 |
市場調查報告書
商品編碼
1273372
血管內導管市場 - COVID-19 的增長、趨勢、影響和預測 (2023-2028)Intravascular Catheter Market - Growth, Trends, and Forecasts (2023 - 2028) |
||||||
※ 本網頁內容可能與最新版本有所差異。詳細情況請與我們聯繫。
預計在預測期內,血管內導管市場的複合年增長率為 9.0%。
COVID-19 大流行對所研究的市場產生了重大影響。 突然爆發的大流行最初對市場產生了短期的負面影響,因為到醫院和診所就診的患者減少了。 然而,由於科學家和研究人員對靜脈導管治療的試驗及其對 COVID-19 患者的相關益處,市場在大流行後階段顯示出相當大的增長。 根據 2022 年 3 月發表在《急診護理雜誌》上的一篇研究論文,一項針對中國 COVID-19 感染者的非隨機臨床試驗表明,外周靜脈導管 (IC) 放置取得了令人滿意的結果,且手術時間短。 因此,這些大流行晚期病例刺激了對靜脈導管放置的需求,最終為最終用戶創造了潛水機會。 因此,儘管市場最初因治療 COVID-19 患者的令人滿意的結果和臨床意義而遭遇挫折,但對 IC 的需求仍在增加。 因此,對 IC 的需求預計將保持其強勢地位並促進市場增長。
目標疾病病例的增加、IC 技術的快速改進以及導管室數量的增加等因素預計將在預測期內推動市場增長。
IC 可以方便地接觸患者的血液,減少對靜脈針的需求。 這使得 IC 成為管理嚴重慢性病患者的重要醫療設備。 血管疾病的增加以及具有更好性能和臨床意義的技術先進產品的可用性是加速研究市場的其他一些因素。
例如,FDA 於 2023 年 1 月發布的數據顯示,心髒病每年影響美國十分之一的成年人,即約 3000 萬人,涵蓋大多數種族和民族。是主要原因之一人的死亡。 此外,根據 2022 年 4 月發表在《化學工程雜誌》上的研究結果,與血管疾病患病率上升相關的住院率上升正在加速 IC 的使用。 IC 可以提供血管診斷、藥物注射和其他手術設備。 因此,預計全球目標疾病的增加將增加對 IC 的需求,這有望進一步促進整體市場的增長。
此外,新產品的發布和技術進步預計將推動所研究市場的增長。 例如,2022 年 9 月,Wipro GE Healthcare 推出了“印度製造”、“人工智能驅動”的 Cath 實驗室 Optima IGS 320,利用 GE 專有的 AutoRight 技術,推進印度的心臟護理。 AutoRight 是第一個由人工智能驅動的基於神經網絡的介入圖像鏈。 實時自動優化圖像和劑量參數。 預計這種市場發展將推動市場的增長。
然而,與導管相關的並發症和副作用以及缺乏醫療報銷預計會阻礙市場增長。
血管內導管市場趨勢
短外周血管內導管 (PIVC) 預計在預測期內顯著增長
一般來說,外周靜脈導管 (PIVC) 是一根用針頭插入靜脈的細塑料管。 短 PIVC 是一種縮短的導管,其尖端插入前臂的小靜脈中。 此外,短 PIVC 是一種套針導管,在導管內放置一根空心金屬針,通常插入淺表靜脈。 短 PIVC 通常稱為插管、Benflon、點滴或點滴。 這些用於大多數臨床環境。
與短 PIVC 相關的優勢和好處預計會贏得最終用戶的需求。 根據 2022 年 3 月發表在 NursingOpen Journal 上的一項研究,該研究開發了一種標準化的 PIVC 審核工具來審核需要插入 PIVC 的住院患者,大多數被審核的短 PIVC 是在病房(57.6%)、手術室(18.7%)中插入的) 和急診科 (11.6%),無需在插入部位進行日常敷料。 因此,高設備採用率預計將有助於這一細分市場的增長。
此外,市場參與者正在致力於產品擴張,這也是預計在預測期內推動該細分市場增長的因素之一。 例如,市場上最短的 BD Insight-N Autoguard Straight IC 等產品的可用性為 14 毫米,可容納小靜脈並允許更多的插入部位選擇。 它也被推薦用於脆弱的靜脈和低靜脈壓的情況。 因此,此類產品在市場上的可用性預計會在參與者之間產生競爭,並有助於該細分市場的增長。
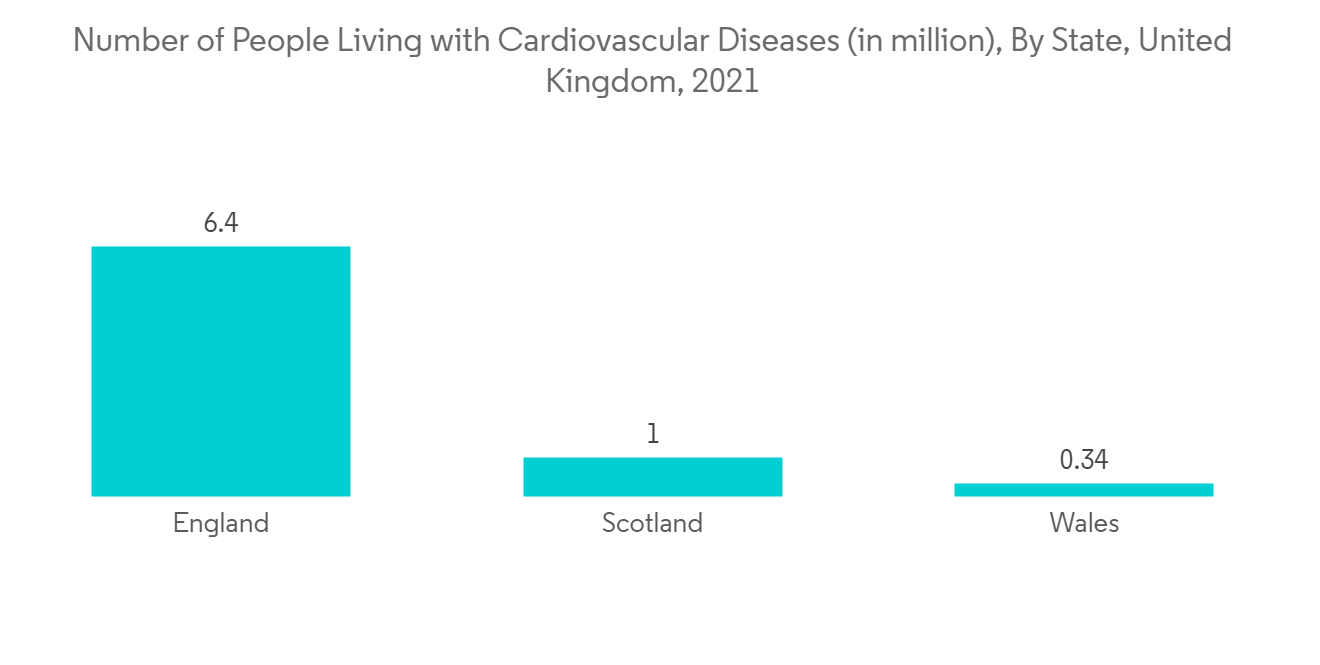
預計北美將佔據很大的市場份額
預計北美將佔據主要市場份額,預計在預測期內將繼續保持類似的增長趨勢。 慢性病患病率上升和產品批准數量增加是推動該地區市場增長的主要因素。
醫療保健疾病是造成醫療保健負擔的主要原因之一。 根據加州大學 2023 年 1 月的更新,心血管或血管疾病是美國男性和女性殘疾和死亡的主要原因。 此外,根據加拿大衛生部 2022 年 7 月公佈的數據,每年 20 歲及以上的加拿大成年人中,約有十分之一(260 萬)患有心髒病。 預計如此高的患病率將增加在血管導管插入術和靜脈通路之後需要干預的醫院入院率。 因此,預計它將推動該地區的市場增長。
此外,一些市場參與者正在實施產品創新等戰略活動,這也有助於該地區的市場增長。 例如,2022 年 4 月,總部位於加利福尼亞州的 Shockwave Medical 在獲得 CE 標誌和食品藥品監督管理局 (FDA) 批准後推出了 Shockwave M5+ 外周血管內碎石術 (IVL) 導管。 同樣在 2022 年 7 月,Intravascular Imaging Incorporated 宣布了用於人類冠狀動脈成像的 3Fr NIRF-IVUS(近紅外熒光血管內超聲)成像導管。 該導管是與馬薩諸塞州總醫院合作設計的,已經在人體冠狀動脈中進行了體外測試,並在臨床前研究中進行了體內測試。 預計這種市場競爭將極大地促進該地區對 IC 的需求擴大。 因此,由於上述因素,預計市場在預測期內將出現顯著增長。
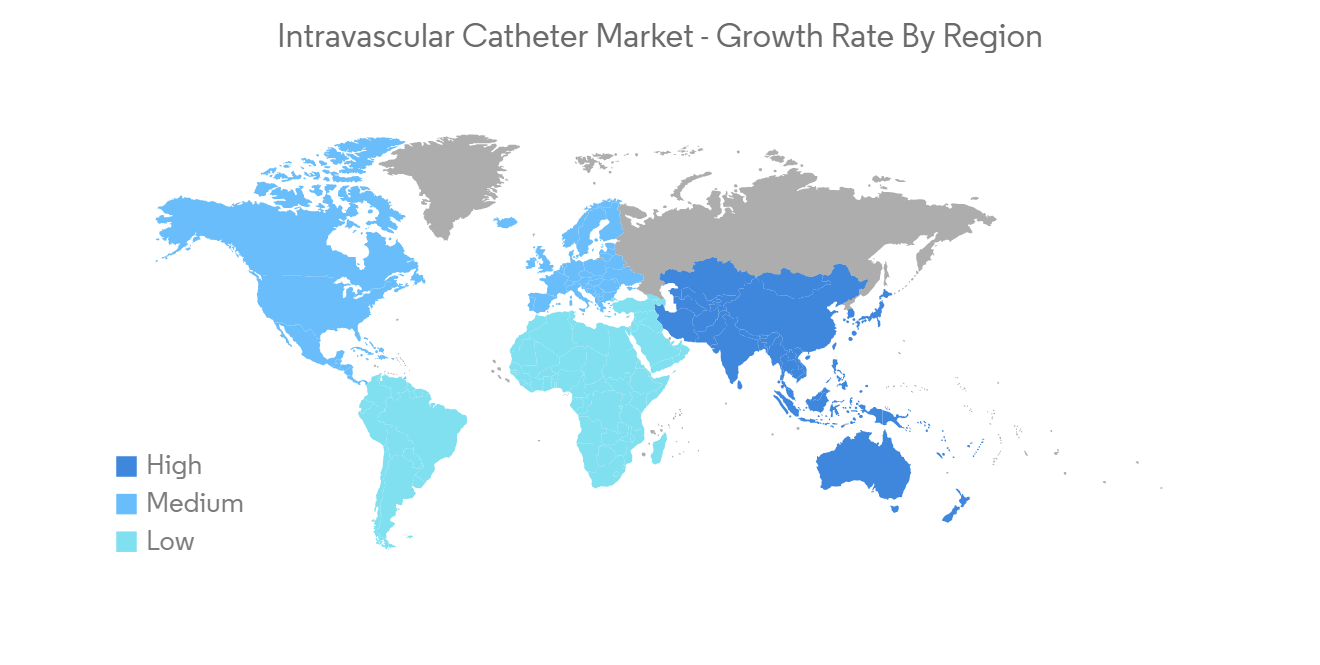
血管內導管行業概況
市場競爭激烈,由從事醫療器械製造、原材料供應、銷售、分銷和其他服務等活動的眾多參與者組成。 因此,投資新興國家的企業數量不斷增加,推動了新興國家市場的增長。 此外,市場受到主要市場參與者之間正在進行的併購的影響。 Boston Scientific Corporation、Becton, Dickinson & Company (C. R. Bard, Inc)、B. Barun、Cook Medical 和 McKesson Corporation 是主要參與者。
其他福利:
- Excel 格式的市場預測 (ME) 表
- 3 個月的分析師支持
內容
第一章介紹
- 調查假設和市場定義
- 本次調查的範圍
第二章研究方法論
第 3 章執行摘要
第四章市場動態
- 市場概覽
- 市場驅動因素
- 心血管疾病和泌尿系統疾病等目標疾病病例增加
- 血管內導管技術的快速改進
- 增加導管實驗室的數量
- 市場製約因素
- 導管相關的副作用
- 缺乏保險報銷
- 波特五力
- 新進入者的威脅
- 買方/消費者議價能力
- 供應商的議價能力
- 替代品的威脅
- 競爭公司之間的敵對關係
第 5 章市場細分
- 按產品分類
- 短外周血管內導管 (PIVC)
- 集成/封閉式 PIVC
- 通過使用
- 腫瘤科
- 胃腸病學
- 心髒病學
- 泌尿科
- 其他用途
- 最終用戶
- 醫院和診所
- 家庭護理
- 其他
- 按地區
- 北美
- 美國
- 加拿大
- 墨西哥
- 歐洲
- 德國
- 英國
- 法國
- 意大利
- 西班牙
- 其他歐洲
- 亞太地區
- 中國
- 日本
- 印度
- 澳大利亞
- 韓國
- 其他亞太地區
- 中東和非洲
- 海灣合作委員會
- 南非
- 其他中東和非洲地區
- 南美洲
- 巴西
- 阿根廷
- 其他南美洲
- 北美
第六章競爭格局
- 公司簡介
- Boston Scientific Corporation
- Becton, Dickinson and Company(C. R. Bard, Inc)
- Medline Industries, Inc
- Cook Medical
- Edwards Life Sciences Corporation
- Johnson & Johnson
- McKesson Corporation
- Medtronic PLC
- Smiths Medical
- Terumo Corporation
- B. Barun
- Angiplast Pvt. Ltd.
第七章市場機會與未來趨勢
The intravascular catheter market is expected to register a CAGR of 9.0% over the forecast period.
The COVID-19 pandemic significantly impacted the market studied. Due to the sudden onset of the pandemic, a short-term negative impact was initially seen on the market, owing to the decreased patient visits to hospitals and clinics. However, due to trials by scientists or researchers in intravenous catheterization and associated benefits among COVID-19 patients, the market witnessed considerable growth in the post-pandemic phase. As per a research article published in the Journal of Emergency Nursing in March 2022, when a nonrandomized clinical trial was conducted among COVID-19-infected patients in China, peripheral intravenous catheter (IC) placement using an infrared vein visualizer showed satisfactory results in a shortened procedure time. Thus, these instances during the later phases of the pandemic created a demand for intravenous catheterization, ultimately generating opportunities for the dives among end users. Therefore, even though the market experienced a setback initially due to its satisfactory outcome and clinical significance in the treatment of COVID-19 patients, the demand for the IC increased. Thus, the demand for ICs is expected to continue its stronghold and contribute to market growth over the coming years.
Factors such as increasing cases of target diseases, rapid improvement in IC technology, and a rising number of catheterization laboratories are anticipated to fuel the market growth during the forecast period.
The ICs allow access to the patient's bloodstream easily and reduce the need for needle sticks into the vein. Due to this, ICs have become vital medical devices for managing patients with severe and chronic diseases. The rise in vascular disease and the availability of technologically advanced products with better performance and clinical significance are a few other factors that are accelerating the studied market.
For instance, as per data released by FDA in January 2023, in the United States, heart disease affects more than 1 in 10 adults, or nearly 30 million, every year and is one of the major causes of mortality for people of most racial and ethnic groups. In addition, as per a research study published in Chemical Engineering Journal in April 2022, the increasing rate of hospitalization associated with the elevated prevalence of vascular diseases has accelerated the use of ICs. The ICs allow vascular diagnostics, injection of drug fluids, and delivery of other surgical devices. Hence, the increase in target disease globally is anticipated to increase the demand for ICs and is further expected to contribute to the overall market growth.
Furthermore, the launch of new products and technological advancements are predicted to drive the studied market growth. For instance, in September 2022, Wipro GE Healthcare launched its 'Made in India,' 'AI-powered' Cath lab-Optima IGS 320 to advance cardiac care in India and leverages the GE proprietary AutoRight technology. AutoRight is the first neural network-based interventional image chain featuring artificial intelligence. It automatically optimizes image and dose parameters in real time. Thus, such developments are likely to support market growth.
However, complications and side effects associated with catheters and a lack of reimbursements are predicted to hinder the market's growth.
Intravascular Catheter Market Trends
Short Peripheral Intravascular Catheters (PIVC) are Expected to Grow Significantly During the Forecast Period
Generally, a peripheral intravenous catheter (PIVC) is a thin plastic tube inserted into a vein using a needle. A short PIVC is a catheter of reduced length that is inserted in the forearm with its tip in a small-caliber vein. Additionally, short PIVCs are over-the-needle catheters that have a hollow metal needle positioned inside the catheter and are generally inserted in superficial veins. The short PIVCs are often called cannulas, venflons, IVs, or drips. These are used in most clinical settings.
The advantages and benefits associated with the short PIVC are anticipated to gain demand among the end users. As per a study published in NursingOpen Journal in March 2022, when a standardized PIVC audit tool was developed to audit hospitalized patients requiring a PIVC insertion, the majority of audited short PIVC were inserted in a ward (57.6%), operating theatre (18.7%) or Emergency Department (ED) (11.6%) without a need for daily dressing on the insertion site. Thus, the high adoption of the devices is anticipated to contribute to the segment's growth.
Additionally, the engagement of market players in product expansion is another factor that is expected to drive the growth of the segment over the forecast period. For instance, the availability of products such as BD Insyte-N Autoguard straight ICs, 14 mm long and the shortest on the market, accommodates small veins and provides a greater choice of insertion sites. Also, these are recommended for fragile veins and low venous pressure. Thus, the availability of such products in the market is anticipated to create competitiveness among the players and contribute to the segment's growth.

North America is Expected to Hold a Significant Share of the Market
North America is expected to hold a major share of the market, and it is predicted to continue the same growth trend over the forecast period. The major factors fuelling the market growth in the region are the increasing prevalence of chronic diseases and the growing number of product approvals.
Cardiovascular disease is one of the major causes of healthcare burden. According to the January 2023 update by the University of California, cardiovascular disease or disorders of blood vessels is the foremost cause of disability and mortality among men and women in the United States. In addition, as per July 2022 data released by Health Canada, about 1 in 12 (or 2.6 million) Canadian adults age 20 and over live with diagnosed heart disease every year. This high prevalence of diseases is anticipated to increase hospital admissions requiring treatments followed by vascular catheterization and vein access. Thereby, it is expected to drive market growth in the region.
Furthermore, several market players are engaged in implementing strategic initiatives such as product innovations and other activities, which are also contributing to the market's growth in the region. For instance, in April 2022, Shockwave Medical, a California-based company, launched its Shockwave M5+peripheral intravascular lithotripsy (IVL) catheter post receiving a CE mark and Food and Drug Administration (FDA) clearance. In addition, in July 2022, Intravascular Imaging Incorporated launched its 3 Fr NIRF-IVUS (Near-Infrared Fluorescence Intravascular Ultrasound) imaging catheter for human coronary imaging. In collaboration with the Massachusetts General Hospital, the catheter was designed and tested ex-vivo in human coronary arteries and in vivo in preclinical studies. These developments are expected to significantly contribute to the competitiveness in the market, augmenting the demand for ICs within the region. Hence, the market is expected to witness significant growth during the forecast period due to the abovementioned factors.

Intravascular Catheter Industry Overview
The market is highly competitive and comprises many players engaged in activities such as medical device manufacturing, raw material supply, sales and distribution, and many other services. Therefore there is an increase in the number of companies investing in emerging nations that drives the market growth in such regions. The market is also influenced by ongoing mergers and acquisitions among major market players. Boston Scientific Corporation, Becton, Dickinson and Company (C. R. Bard, Inc), B. Barun, Cook Medical, and McKesson Corporation are a few major players.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Cases of Target Diseases Such as Cardiovascular, Urological and Other Diseases
- 4.2.2 Rapid Improvement in Intravascular Catheter Technology
- 4.2.3 Increasing Number of Catheterization Laboratories
- 4.3 Market Restraints
- 4.3.1 Side effects Associated with the Catheters
- 4.3.2 Lack of Reimbursement
- 4.4 Porter Five Forces
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION
- 5.1 By Product
- 5.1.1 Short Peripheral Intravascular Catheters (PIVC)
- 5.1.2 Integrated/Closed PIVC
- 5.2 By Application
- 5.2.1 Oncology
- 5.2.2 Gastroenterology
- 5.2.3 Cardiology
- 5.2.4 Urology
- 5.2.5 Other Applications
- 5.3 By End User
- 5.3.1 Hospitals and Clinics
- 5.3.2 Homecare Setting
- 5.3.3 Others
- 5.4 Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Boston Scientific Corporation
- 6.1.2 Becton, Dickinson and Company (C. R. Bard, Inc)
- 6.1.3 Medline Industries, Inc
- 6.1.4 Cook Medical
- 6.1.5 Edwards Life Sciences Corporation
- 6.1.6 Johnson & Johnson
- 6.1.7 McKesson Corporation
- 6.1.8 Medtronic PLC
- 6.1.9 Smiths Medical
- 6.1.10 Terumo Corporation
- 6.1.11 B. Barun
- 6.1.12 Angiplast Pvt. Ltd.







