 |
市場調查報告書
商品編碼
1273409
神經血管通路導管市場 - 增長、趨勢、COVID-19 影響和預測 (2023-2028)Neurovascular Access Catheters Market - Growth, Trends, and Forecasts (2023 - 2028) |
||||||
※ 本網頁內容可能與最新版本有所差異。詳細情況請與我們聯繫。
在預測期內,神經血管通路導管市場預計將以 4.9% 的複合年增長率增長。
COVID-19 對神經血管通路導管市場產生了重大影響,因為在第一波大流行期間許多神經血管手術不得不推遲或取消。 例如,2021 年 2 月發表在 PubMed 上的一篇論文發現,冠狀病毒會影響神經系統表現和健康,神經系統患者需要更頻繁地住院和出院。這是因為它增加了死亡和殘疾的風險。 由於全球封鎖,神經系統疾病患者很難接受定期檢查和治療,這對所研究的市場產生了重大影響。 但現在限制已經解除,神經管問題變得越來越普遍,越來越多的人使用神經血管通路導管,因此市場可能會增長。
預計市場增長將受到神經血管疾病增加和神經血管導管技術改進等因素的推動。 例如,根據 2022 年 3 月的前沿文章,全球蛛網膜下腔出血 (SAH) 的估計發病率為每 100,000 人 6.67 例。 每年,SAH 影響約 500,000 人,其中約三分之二生活在低收入和中等收入國家。 此外,神經血管通路導管的技術進步預計將在預測期內推動市場增長。 例如,2022 年 2 月,CERENOVUS 是一家專注於神經血管護理的初創公司,隸屬於強生醫療器械公司,該公司宣布推出其下一代球囊導管,用於血管內手術,包括急性缺血性中風患者。導管“ EMBOGUARD”發布。
此外,研究和比較不同的神經血管通路導管應該會在研究期間促進市場的增長。 例如,2022 年 9 月發表在 PubMed 上的一篇論文指出,內徑為 0.096 英寸的通路導管可用於通過股動脈和橈動脈進行神經血管手術。 技術成功率高,治療過程中故障發生率低。
簡而言之,神經血管並發症的惡化、更多的神經血管通路導管產品以及研發的增加預計將在未來幾年擴大研究中的市場。 然而,生物相容性問題可能會減緩神經血管通路導管的市場增長。
神經血管通路導管市場趨勢
預計在預測期內,多腔導管部分將在神經血管通路導管市場中佔據顯著的市場份額
多腔導管是□□具有多個內部通道(稱為腔)的單個導管。 每個內腔都可以連接到不同的靜脈注射,並且液體通常會沿著導管在略有不同的位置排出。 由於缺血性中風、狹窄和腦動脈瘤等神經血管疾病患病率上升以及神經血管導管技術進步等因素,預計多腔導管市場將在預測期內擴大。 例如,根據“2022 年全球中風情況說明書”,2022 年全球將報告 12,224,551 例缺血性中風。 根據同一消息來源,每年有超過 1220 萬例新中風發生。 在全球範圍內,25 歲以上的人中有四分之一會在一生中患中風。 因此,預計缺血性中風病例和使用多腔導管進行治療會增加,從而推動市場增長。
此外,預計主要製造商增加產品發布、戰略聯盟和合作夥伴關係將推動市場增長。 例如,Zeus Industrial Products 於 2022 年 2 月在其產品線中添加了 PTFE Sub-Lite-Wall 多腔管。 它具有超薄壁,通過了 USP VI 級生物相容性認證,具有高結構完整性、改進的平面度、高潤滑性和出色的介電強度。
因此,由於神經血管並發症的增加、多腔導管產品發布的增加以及研發的增加,預計所研究的細分市場將在預測期內呈現增長。
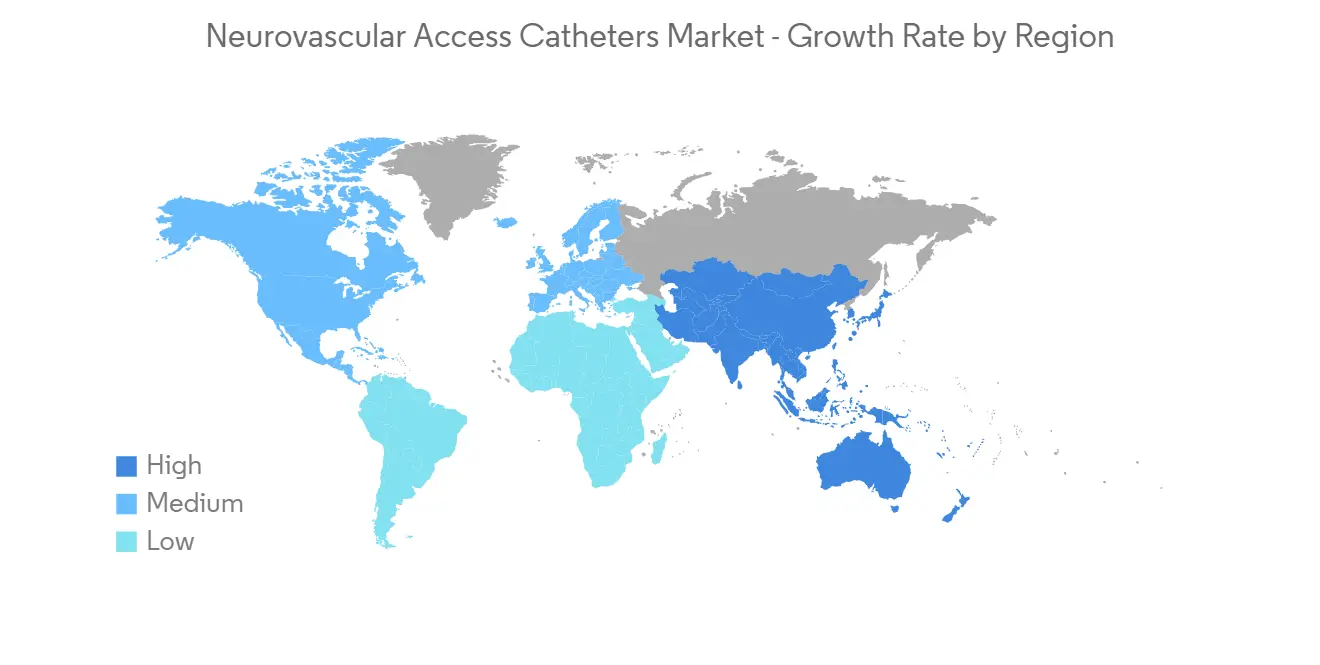
預計北美將佔據很大的市場份額,預計在預測期內也會如此
預計北美將佔據全球神經血管通路導管市場的很大份額。 這是由於該地區缺血性中風、狹窄和腦動脈瘤等神經血管疾病的流行,神經血管導管插入術的流行率越來越高,診斷率高。
腦動脈瘤基金會 2023 年更新估計,美國估計有 670 萬人患有未破裂的腦動脈瘤,其中每 50 人中就有 1 人患有未破裂的腦動脈瘤。 在美國,每年約有 30,000 人患有腦動脈瘤破裂。 每 18 分鐘就有一個腦動脈瘤破裂。 因此,腦動脈瘤的高患病率可能會增加神經血管通路導管的採用,從而促進市場增長。 此外,不斷增加的產品發布和完善的醫療保健基礎設施的存在也為整個區域市場的增長做出了重大貢獻。 例如,2022 年 8 月,神經血管設備開發商 Vena Medical 在倫敦健康科學中心(LHSC)、大學醫院和渥太華醫院(TOH)宣布全球首例使用 Vena BDAC 治療 5 名患者。 加拿大將率先受益於在患者後院開發的 Vena BDAC 技術,該技術適用於需要機械血栓切除術治療缺血性中風的患者。 血栓切除術是一種去除血塊的微創手術,現在是大血管閉塞引起的急性缺血性中風 (AIS) 患者的標準治療方法。
隨著神經血管疾病變得更加普遍、神經血管通路導管產品的增加以及研發的進步,預計未來幾年北美將實現增長。
神經血管通路導管行業概況
神經血管通路導管市場競爭適中,由幾家大型企業組成。 就市場份額而言,目前少數大公司佔據市場主導地位。 Stryker Corporation、Medtronic Plc、Johnson & Johnson、Integer Holdings Corporation、Biomerics、Penumbra, Inc、Zeus Industrial Products, Inc 和 Imperative Care Inc 是市場領導者。
其他福利:
- Excel 格式的市場預測 (ME) 表
- 3 個月的分析師支持
內容
第一章介紹
- 調查假設和市場定義
- 本次調查的範圍
第二章研究方法論
第 3 章執行摘要
第四章市場動態
- 市場概覽
- 市場驅動力
- 神經血管疾病的患病率增加
- 神經血管導管技術進展
- 市場製約因素
- 神經血管通路導管中存在生物相容性問題
- 波特的五力分析
- 新進入者的威脅
- 買方/消費者議價能力
- 供應商的議價能力
- 替代品的威脅
- 競爭公司之間的敵對關係
第 5 章市場細分
- 按產品類型
- 單腔導管
- 雙腔導管
- 多腔導管
- 按材料
- 液晶聚合物
- 氟化乙烯丙烯
- 其他材料
- 按地區
- 北美
- 美國
- 加拿大
- 墨西哥
- 歐洲
- 德國
- 英國
- 法國
- 意大利
- 西班牙
- 其他歐洲
- 亞太地區
- 中國
- 日本
- 印度
- 澳大利亞
- 韓國
- 其他亞太地區
- 中東和非洲
- 海灣合作委員會
- 南非
- 其他中東和非洲地區
- 南美洲
- 巴西
- 阿根廷
- 其他南美洲
- 北美
第六章競爭格局
- 公司簡介
- Stryker Corporation
- Medtronic Plc
- Johnson & Johnson
- Integer Holdings Corporation
- Biomerics
- Penumbra, Inc
- Zeus Industrial Products, Inc
- Imperative Care Inc
- ICU Medical, Inc
- Teleflex Incorporated
第七章市場機會與未來趨勢
During the time frame of the forecast, the neurovascular access catheters market is expected to grow at a CAGR of 4.9%.
COVID-19 had a big effect on the market for neurovascular access catheters because many neurovascular surgeries had to be put off or stopped during the first wave of the pandemic. For example, a February 2021 article in PubMed says that coronavirus affects neurological presentations and health status because neurological patients need to stay in the hospital more often and have a higher risk of death and disability when they get out.Due to the global lockdown, it was hard for patients with neurological problems to get regular checks and treatment, which had a big effect on the market studied.But now that the restrictions have been lifted, the market is likely to grow because neurovascular problems are becoming more common and more people are using neurovascular access catheters.
Market growth is likely to be driven by things like the rise of neurovascular diseases and the improvement of neurovascular catheter technology.For example, according to an article in Frontiers from March 2022, the estimated global incidence of subarachnoid hemorrhage (SAH) is 6.67 per 100,000 people. Each year, SAH affects nearly 500,000 people, and nearly two-thirds of these people live in low- and middle-income countries.Furthermore, technological advances in neurovascular access catheters are expected to drive market growth over the forecast period. For instance, in February 2022, CERENOVUS, an emerging company that focuses on neurovascular care and is part of the Johnson & Johnson Medical Device Companies, launched the EMBOGUARD, a next-generation balloon guide catheter to be used in endovascular procedures, including those for patients with acute ischemic stroke.
Also, research studies and comparisons of the different neurovascular access catheters should help the market grow during the study period. For example, a September 2022 article in PubMed said that a 0.096-inch inner diameter access catheter could be used for neurovascular treatments through both the femoral artery and the radial artery. It had a good rate of technical success and a low rate of problems during the procedure.
So, the studied market is likely to grow over the next few years because neurovascular complications are getting worse, more neurovascular access catheter products are coming out, and more research and development is being done. But problems with biocompatibility are likely to slow market growth for neurovascular access catheters.
Neurovascular Access Catheters Market Trends
Multiple Lumen Catheters Segment is Expected to Hold a Notable Market Share in the Neurovascular Access Catheters Market Over the Forecast Period
A multi-lumen catheter is a single catheter that has multiple internal channels (called lumens). Each lumen can be connected to a distinct intravenous infusion, and the fluid will normally emerge at a slightly different place along the catheter. The multi-lumen catheter market is expected to expand during the forecast period due to factors such as the rising prevalence of neurovascular disorders such as ischemic stroke, stenosis, brain aneurysm, and other illnesses, as well as technological advancements in neurovascular catheters. For instance, as per the Global Stroke Fact Sheet 2022, 12,224,551 cases of ischemic stroke were reported in 2022 across the globe. As per the same source, there are over 12.2 million new strokes each year. Globally, one in four people over age 25 will have a stroke in their lifetime. Hence, the rise in cases of ischemic stroke is anticipated, utilizing the multi-lumen catheter for the treatment and thereby promoting the market growth.
Additionally, an increase in product launches, strategic collaborations, and partnerships by the major players is expected to promote market growth. For example, Zeus Industrial Products added PTFE Sub-Lite-Wall multi-lumen tubing to its line of products in February 2022.It has ultrathin walls, is certified USP Class VI biocompatible, has high structural integrity, improved planarity, high lubricity, and excellent dielectric strength.
Thus, due to the rise in neurovascular complications, increase in multi-lumen catheter product launches, and increase in research and development, the studied segment is anticipated to witness growth over the forecast period.

North America is Expected to Hold a Significant Share in the Market and Expected to do Same in the Forecast Period
North America is expected to have a large share of the global market for neurovascular access catheters. This is because neurovascular disorders like ischemic stroke, stenosis, brain aneurysms, and other illnesses are becoming more common, neurovascular catheters are becoming more popular, and the rate of diagnosis is high in this region.
As per the Brain Aneurysm Foundation's 2023 update, an estimated 6.7 million people in the United States have an unruptured brain aneurysm, or 1 in 50 people. About 30,000 people in the United States suffer a brain aneurysm rupture each year. A brain aneurysm ruptures every 18 minutes. Hence, the high prevalence of brain aneurysms is likely to increase the adoption of neurovascular access catheters and thereby boost market growth. Furthermore, increasing product launches and the presence of a well-established healthcare infrastructure are also fueling the growth of the overall regional market to a large extent. For instance, in August 2022, Vena Medical, a developer of neurovascular devices, publicized the successful treatment of the first five patients in the world using the Vena BDAC at the London Health Sciences Centre (LHSC), the University Hospital, and The Ottawa Hospital (TOH). Canada is the first to benefit from the Vena BDAC technology developed right in the patient's backyard for those requiring mechanical thrombectomy during ischemic stroke treatment. Thrombectomy, a minimally invasive procedure to remove a blood clot, is now the standard of care treatment for patients with an acute ischemic stroke (AIS) secondary to a large vessel occlusion.
North America is expected to grow over the next few years because neurovascular diseases are becoming more common, more neurovascular access catheter products are coming out, and more research and development is being done.

Neurovascular Access Catheters Industry Overview
The neurovascular access catheters market is moderately competitive and consists of several major players. In terms of market share, a few of the major players are currently dominating the market. Stryker Corporation, Medtronic Plc, Johnson & Johnson, Integer Holdings Corporation, Biomerics, Penumbra, Inc, Zeus Industrial Products, Inc, and Imperative Care Inc are some of the market leaders.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Prevalence of Neurovascular Disorders
- 4.2.2 Technological Advancements in Neurovascular Catheters
- 4.3 Market Restraints
- 4.3.1 Presence of Biocompatibility Issues with Neurovascular Access Catheters
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD Million)
- 5.1 By Product Type
- 5.1.1 Single Lumen Catheters
- 5.1.2 Double Lumen Catheters
- 5.1.3 Multiple Lumen Catheters
- 5.2 By Material
- 5.2.1 Liquid Crystal Polymer
- 5.2.2 Fluorinated Ethylene Propylene
- 5.2.3 Other Materials
- 5.3 Geography
- 5.3.1 North America
- 5.3.1.1 United States
- 5.3.1.2 Canada
- 5.3.1.3 Mexico
- 5.3.2 Europe
- 5.3.2.1 Germany
- 5.3.2.2 United Kingdom
- 5.3.2.3 France
- 5.3.2.4 Italy
- 5.3.2.5 Spain
- 5.3.2.6 Rest of Europe
- 5.3.3 Asia-Pacific
- 5.3.3.1 China
- 5.3.3.2 Japan
- 5.3.3.3 India
- 5.3.3.4 Australia
- 5.3.3.5 South Korea
- 5.3.3.6 Rest of Asia-Pacific
- 5.3.4 Middle East and Africa
- 5.3.4.1 GCC
- 5.3.4.2 South Africa
- 5.3.4.3 Rest of Middle East and Africa
- 5.3.5 South America
- 5.3.5.1 Brazil
- 5.3.5.2 Argentina
- 5.3.5.3 Rest of South America
- 5.3.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Stryker Corporation
- 6.1.2 Medtronic Plc
- 6.1.3 Johnson & Johnson
- 6.1.4 Integer Holdings Corporation
- 6.1.5 Biomerics
- 6.1.6 Penumbra, Inc
- 6.1.7 Zeus Industrial Products, Inc
- 6.1.8 Imperative Care Inc
- 6.1.9 ICU Medical, Inc
- 6.1.10 Teleflex Incorporated













