 |
市場調查報告書
商品編碼
1349828
HER2 低表達胃或胃食道交界處 (GEJ) 腺癌市場:初步研究 (KOL 見解) - 市場情報 - 流行病學和 2034 年市場預測HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma | Primary Research (KOL's Insight) | Market Intelligence | Epidemiology & Market Forecast-2034 |
||||||
化療、免疫療法和標靶治療對 HER2 低表達胃或胃食道連接部 (GEJ) 腺癌市場貢獻顯著。 到 2034 年,新興療法的採用預計將成為一個主要轉捩點,導致 HER2 低表達胃或胃食道連接處 (GEJ) 腺癌藥物市場發生巨大變化。
專家認為,胃癌和其他一些癌症表達 HER2,並對□□ HER2 標靶治療有反應。 因此,人們對研究 T-DXd 在這些癌症中的應用越來越感興趣,特別是在 HER2 表達低的患者中。
胃和胃食道連接部 (GEJ) 腺癌是侵襲性惡性腫瘤,治療選擇有限。 HER2 低表達腫瘤定義為免疫組織化學 (IHC) 分析得分為 1+ 或原位雜交 (ISH) 陰性且 IHC 得分為 2+。 這種腫瘤亞型在胃或 GEJ 腺癌中的特徵很差,其臨床意義尚不清楚。 然而,最近的證據表明 HER2 低表達可能會影響預後並指導治療決策。
HER2 低表達的胃或胃食道交界部腺癌患者在治療選擇方面面臨挑戰。 傳統的 HER2 標靶療法,例如曲妥珠單抗,在該亞組中顯示出有限的療效。 因此,應考慮替代治療策略以改善這些患者的預後。
本報告調查了全球 HER2 低表達胃或胃食道交界處 (GEJ) 腺癌市場,包括市場現狀、病例數趨勢、患者趨勢、競爭產品的市場定位,並為我們提供機會例如
目錄
第 1 章執行摘要
第2章 HER2低表達胃或胃食道結合部(GEJ)腺癌的疾病背景
- HER2 低表達胃或胃食道交界處 (GEJ) 腺癌的定義
- 原因和症狀
- 病理生理學
- 導致 HER2 表達低的因素
第 3 章 HER2 低表達胃或胃食道交界處 (GEJ) 腺癌的診斷
第 4 章目前的治療方法與醫療實務
- 主要發現
- 處理演算法
第 5 章未滿足的需求
第6章新的治療方法
- 主要發現
- 管道概覽
- HER2 低表達胃或胃食道交界處 (GEJ) 腺癌領域取得顯著進展
第 7 章 HER2 低表達胃或胃食道交界處 (GEJ) 腺癌上市時間表和主要市場事件
第 8 章 HER2 低表達胃或胃食道交界處 (GEJ) 腺癌 - 定價與報銷
第 9 章 KOL 洞察(美國、歐盟、日本)
第10章未來治療典範
- HER2 低表達胃或胃食道交界處 (GEJ) 腺癌的競爭格局和批准
- 未來的治療演算法與競爭定位
- 新療法的重要數據摘要
- 當前和未來治療的年度費用
- HER2低表達胃或胃食道交界處 (GEJ) 腺癌晚期治療的策略考慮
第11章市場展望
- 主要發現
- 摘要
- 2034 年之前按國家劃分的市場預測
第12章市場促進與約束因子
第13章附錄
第14章研究方法
The HER2-Low Gastric / Gastroesophageal Junction (GEJ) Adenocarcinoma market is hugely contributed by chemotherapy, immunotherapy, and targeted therapies. By 2034, the uptake of novel emerging therapies will serve as a major breakpoint to get a drastic change in the HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma therapeutics market.
"Experts believe that gastric cancer and some other cancers express HER2 and respond to HER2-targeted therapies. This has led to a growing interest in studying the use of T-DXd in these cancers, particularly in patients with low expression of HER2."
Gastric and gastroesophageal junction (GEJ) adenocarcinomas are aggressive malignancies with limited treatment options. HER2-low tumors are defined as having a score of 1+ on immunohistochemical (IHC) analysis or an IHC score of 2+ with negative results on in situ hybridization (ISH). This subset of tumors has not been well characterized in gastric or GEJ adenocarcinomas, and their clinical significance remains unclear. However, recent evidence suggests that HER2-low expression may have prognostic implications and could potentially guide treatment decisions.
Patients with HER2 low gastric or gastroesophageal junction adenocarcinoma pose a challenge in terms of treatment selection. Traditional HER2-targeted therapies, such as trastuzumab, have shown limited efficacy in this subgroup. Therefore, alternative treatment strategies need to be explored to improve outcomes for these patients.
Mellalta's HER2-Low Gastric / Gastroesophageal Junction (GEJ) Adenocarcinoma Report- Market Summary
| Report Attributes | Details |
| Market Size 2034: | $ billion |
| Key Market Players: | Duality Biologics; AstraZeneca/Daiichi Sankyo |
| Forecast Period: | 2020-2034. |
| Countries Covered: | US, France, Germany, Italy, Spain, UK, China and Japan. |
| Current SOC Chemotherapy: | Immunotherapy; Targeted Therapies. |
| Future SOC: | Targeted Therapies; Combination Approach. |
| Key Unmet Need: | Improved HER2 Assessment; Limited Treatment Option. |
| Key Clinical Insights: | The poor prognosis observed in patients with HER2-low gastric or GEJ adenocarcinoma highlights the need for novel treatment approaches and pharmaceutical companies are now exploring the development of novel agents specifically designed to address the needs of this patient population. |
| Provider-Patient (PPP) Perspective: | Cost-effectiveness of treatment; Better Diagnostic Identification; Access to appropriate treatments and improved options. |
Mellalta's HER2-Low Gastric / Gastroesophageal Junction (GEJ) Adenocarcinoma Report - Epidemiology
The total incident cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma in the G7 countries are anticipated to increase by a significant number of cases by 2034 for the study period (2020-2034). As per estimates, the United States will present with the highest incidence of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma cases in 2034. Among the EU5, Germany had the highest HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma cases, followed by the UK, France, Italy, and Spain. Japan is reported to have the highest number of treated cases after the United States, Germany, and the UK.
A retrospective study evaluated HER-2 expression in early-stage gastric cancer using immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) and found that 8.3% of patients had HER2-positive tumors, 31.8% had HER2-low tumors, and 50.3% had HER2-negative tumors.
Mellalta's HER2-Low Gastric / Gastroesophageal Junction (GEJ) Adenocarcinoma Report - Current Market Size & Forecast Trends
The current standard of care is limited to chemotherapy, immunotherapy, and targeted therapy. Recent developments have shown that ADCs, such as DS-8201 and Disitamab vedotin, release intracellular toxins that can exert a killing effect on neighboring cells without target expression. This bystander effect allows patients with HER2 Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma to benefit from HER2-targeted therapy. Therefore, ADCs may expand the population that can benefit from HER2-targeted therapy and are expected to be a novel option for patients whose tumors have low HER2 expression.
The advent of anti-HER2 therapies, such as trastuzumab and trastuzumab deruxtecan (T-DXd), has revolutionized the treatment landscape for HER2-positive gastric or gastroesophageal junction adenocarcinoma. However, the efficacy of these therapies in HER2-low tumors remains uncertain. Recent studies have shown promising results with T-DXd in patients with HER2-low gastric cancer, suggesting that HER2-low tumors may still benefit from HER2-targeted treatments. Based on the results of the DESTINY-Gastric01 trial, T-DXd was approved in Japan for the treatment of patients with HER2-positive unresectable advanced or recurrent gastric cancer that has progressed after chemotherapy. T-DXd is now recommended in the Japanese Gastric Cancer Association treatment guidelines as the third-line treatment for HER2-positive gastric cancer. Additional studies on T-DXd for the treatment of AGC are also ongoing. The DESTINY-Gastric02 trial (NCT04014075) is an open-label, single-arm phase II trial to assess the efficacy and safety of T-DXd in HER2-positive AGC patients who have been treated with previous trastuzumab containing chemotherapy in non-Asian populations. The primary endpoint is the ORR by independent central review, similar to the DESTINY-Gastric01 trial. Seventy-two patients will be enrolled.
Mellalta Meets Current Treatment Strategies in Gastric Cancer
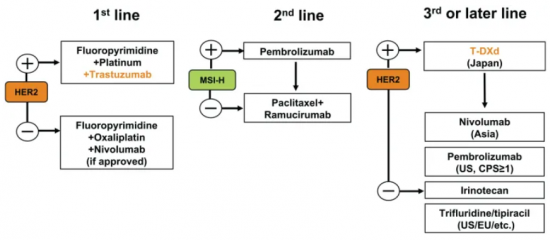
Current Treatment Strategies in Gastric Cancer Source: Daisuke Kotani et al 2021
In the 2024-2034 forecast period, there will be tremendous growth and shift in therapeutic market with the launch of novel emerging therapies like DB-1303 (Duality Biologics), Trastuzumab deruxtecan (AstraZeneca/Daiichi Sankyo), and more. We expect a greater uptake of the new therapies which will result in better treatment outcomes for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma market space. The launch of these upcoming therapies will drive the highly competitive therapeutic market in the coming time.
Questions Answered:
- What is the size of clinically and commercially relevant drug-treatable HER2 low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma populations, and how will drug-treatment rates of HER2 change over time?
- Potential challenges and opportunities in implementing targeted therapies for HER2-low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma.
- What are the most promising agents in the pipeline, and how will they shape the future of this therapy market?
- What key drivers and constraints will affect the HER2 low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma therapy market over the forecast period?
Report Highlights:
- HER2-Low Gastroesophageal Junction (GEJ) Adenocarcinoma - Current Market Trends
- HER2-Low Gastroesophageal Junction (GEJ) Adenocarcinoma - Current & Forecasted Cases across the G7 Countries
- HER2-Low Gastroesophageal Junction (GEJ) Adenocarcinoma - Market Opportunities and Sales Potential for Agents
- HER2-Low Gastroesophageal Junction (GEJ) Adenocarcinoma - Patient-based Market Forecast to 2034
- HER2-Low Gastroesophageal Junction (GEJ) Adenocarcinoma - Untapped Business Opportunities
- HER2-Low Gastroesophageal Junction (GEJ) Adenocarcinoma - Product Positioning Vis-a-vis Competitors' Products
- HER2-Low Gastroesophageal Junction (GEJ) Adenocarcinoma - KOLs Insight
Table of Content
1. Executive Summary
- 1.1. Key Findings
- 1.2. Key Market Challenges and Opportunities
- 1.3. What Do the Experts Say?
2. HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma Disease Background
- 2.1. HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma Definition
- 2.2. Cause & Symptoms
- 2.3. Pathophysiology
- 2.4. Factors contributing to the HER2-Low Expression in Breast Cancer
3. HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma-Diagnosis
- 3.1. HER2 Assessment with Immunohistochemistry (IHC) and In Situ Hybridization (ISH) (ASCO/CAP Guidelines)
- 3.2. Epidemiology and Patient Populations
- 3.3. Key Findings
- 3.4. Methods and data Sources
- 3.4.1. Country Specific Incident cases of Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (US, Germany, France, Italy, Spain, UK, and Japan)
- 3.4.2. Country Specific Incident cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.3. Country Specific Treated cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.4. Key Sources for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma Epidemiology and Model Parameters
- 3.4.4.1. United States
- 3.4.4.1.1. United States Incident cases of Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (US, Germany, France, Italy, Spain, UK, and Japan)
- 3.4.4.1.2. United States Incident cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.4.1.3. United States Treated cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.4.2. Germany
- 3.4.4.2.1. Germany Incident cases of Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (US, Germany, France, Italy, Spain, UK, and Japan)
- 3.4.4.2.2. Germany Incident cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.4.2.3. Germany Treated cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.4.3. France
- 3.4.4.4. France Incident cases of Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (US, Germany, France, Italy, Spain, UK, and Japan)
- 3.4.4.5. France Incident cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.4.6. France Treated cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.4.1. United States
- 3.4.5. Italy
- 3.4.5.1. Italy Incident cases of Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (US, Germany, France, Italy, Spain, UK, and Japan)
- 3.4.5.2. Italy Incident cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.5.3. Italy Treated cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.5.4. Spain
- 3.4.5.4.1. Spain Incident cases of Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (US, Germany, France, Italy, Spain, UK, and Japan)
- 3.4.5.4.2. Spain Incident cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.5.4.3. Spain Treated cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.5.5. United Kingdom
- 3.4.5.5.1. United Kingdom Incident cases of Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (US, Germany, France, Italy, Spain, UK, and Japan)
- 3.4.5.5.2. United Kingdom Incident cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.5.5.3. United Kingdom Treated cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.5.6. Japan
- 3.4.5.6.1. Japan Incident cases of Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma (US, Germany, France, Italy, Spain, UK, and Japan)
- 3.4.5.6.2. Japan Incident cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
- 3.4.5.6.3. Japan Treated cases of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
4. Current Therapy and Medical Practice
- 4.1. Key Findings
- 4.2. Treatment Algorithm
5. Unmet Needs
6. Emerging Therapy
- 6.1. Key Findings
- 6.2. Pipeline Overview
- 6.3. Notable Developments in the HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma space
- 6.3.1. Product Analysis
- 6.3.1.1. DB-1303 (Duality Biologics)
- 6.3.1.1.1. Product Profile
- 6.3.1.1.2. Clinical Development
- 6.3.1.1.3. Market & Sales Opportunity Forecasted to 2034
- 6.3.1.2. Trastuzumab deruxtecan (AstraZeneca/Daiichi Sankyo)
- 6.3.1.2.1. Product Profile
- 6.3.1.2.2. Clinical Development
- 6.3.1.2.3. Market & Sales Opportunity Forecasted to 2034
- 6.3.1.3. Product A (Company A)
- 6.3.1.3.1. Product Profile
- 6.3.1.3.2. Clinical Development
- 6.3.1.3.3. Market & Sales Opportunity Forecasted to 2034
- 6.3.1.4. Product B (Company B)
- 6.3.1.4.1. Product Profile
- 6.3.1.4.2. Clinical Development
- 6.3.1.4.3. Market & Sales Opportunity Forecasted to 2034
- 6.3.1.4.4. Others
- 6.3.1.1. DB-1303 (Duality Biologics)
- 6.3.1. Product Analysis
7. Launch Timeline & Key Market Events for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
8. HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma - Pricing & Reimbursement
9. KOLs Insight (US, EU, JP)
- 9.1. Unmet Needs
- 9.2. Analysis of the progresses in terms of approvals & current pipeline;
- 9.3. Impact on the treatment algorithm and product positioning
- 9.4. Relevance of new targets/platforms/ therapies Uptake Share %
- 9.5. Physicians Preferences for the new therapies
10. Future Treatment Paradigm
- 10.1. HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma Competitor Landscape and Approvals Anticipated
- 10.2. Future Treatment Algorithms and Competitor Positioning
- 10.3. Key Data Summary for Emerging Treatment
- 10.4. Annual Cost of Current & Emerging Treatment
- 10.5. Late Phase Therapy Strategic Considerations in HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma
11. Market Outlook
- 11.1. Key Findings
- 11.2. Overview
- 11.3. Country Specific Market Forecast to 2034
- 11.3.1. Sales of Drugs to Treat HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma in the Major Pharmaceutical Markets, 2020-2034
- 11.3.2. Patient Share of HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma by Therapies
- 11.3.3. Market Forecast by Country
- 11.3.3.1. United States
- 11.3.3.1.1. United States Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma 2020-2034 (USD Million)
- 11.3.3.1.2. United States Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma by Therapy 2020-2034 (USD Million)
- 11.3.3.2. Germany
- 11.3.3.2.1. Germany Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma 2020-2034 (USD Million)
- 11.3.3.2.2. Germany Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma by Therapy 2020-2034 (USD Million)
- 11.3.3.3. France
- 11.3.3.3.1. France Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma 2020-2034 (USD Million)
- 11.3.3.3.2. France Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma by Therapy 2020-2034 (USD Million)
- 11.3.3.4. Italy
- 11.3.3.4.1. Italy Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma 2020-2034 (USD Million)
- 11.3.3.4.2. Italy Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma by Therapy 2020-2034 (USD Million)
- 11.3.3.5. Spain
- 11.3.3.5.1. Spain Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma 2020-2034 (USD Million)
- 11.3.3.5.2. Spain Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma by Therapy 2020-2034 (USD Million)
- 11.3.3.6. United Kingdom
- 11.3.3.6.1. United Kingdom Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma 2020-2034 (USD Million)
- 11.3.3.6.2. United Kingdom Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma by Therapy 2020-2034 (USD Million)
- 11.3.3.7. Japan
- 11.3.3.7.1. Japan Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma 2020-2034 (USD Million)
- 11.3.3.7.2. Japan Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma by Therapy 2020-2034 (USD Million)
- 11.3.3.1. United States
12. Market Drivers and Constraints
- 12.1. What Factors Are Driving the Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma?
- 12.2. What Factors Are Constraining the Market for HER2-Low Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma?





