 |
市場調查報告書
商品編碼
1213704
照護現場分子診斷(mPOC)市場:規模和未來性(2023年)The Market and Future Potential for Molecular Point of Care (mPOC), 2023 |
||||||
本報告提供全球照護現場分子診斷(mPOC)市場相關調查,照護現場分子診斷概要與市場的發展趨勢與應用領域,及加入此市場的主要企業的簡介等資訊。
目錄
第1章 摘要整理
第2章 分子照護現場市場開拓和趨勢
- COVID-19和POC分子診斷
- 最近的法規趨勢
- 照護現場分子診斷的優點與缺點
- POC診斷的一般檢驗和檢體
- 照護現場分子診斷的要素技術
- 微射流
- qPCR
- 微陣列
- 等溫放大
- 試驗的自動化
- 底層塗料和探針
- 檢測
- 次世代定序
- 分子診斷學
- 即時PCR(qPCR)
- 等溫放大法
- 線探針化驗
- 次世代定序
- 照護現場分子診斷的應用
- 照護現場分子診斷的主要實驗
- 流感
- RIDT的再分類
- 院內感染(HAI)
- 鏈球菌A
- 呼吸道融合細胞病毒(RSV)
- 新的用途
- 茲卡熱
- 其他的呼吸系統感染疾病
- B族鏈球菌
- 人類乳突病毒
- 單純皰疹病毒
- 陰道炎
- 結核
- 瘧疾
- 其他的熱帶疾病及受到忽視的疾病
- 癌症
- 歐洲的設備法規
第3章 市場分析
- 照護現場分子診斷市場分析
- 在床邊的分子診斷市場
- 床邊診斷市場佔有率
第4章 企業簡介
- Abbott Laboratories
- Aidian Oy
- Akonni Biosystems
- binx health, inc.
- Biocartis NV
- bioMerieux SA
- Cepheid (Danaher)
- Molecular Healthcare-Acquired Infection (HAI) Testing
- Molecular Sexual Health and Women's Health Testing
- Molecular Critical Infectious Disease Testing
- Molecular Oncology/Genetics Testing
- Credo Diagnostics
- Cue Health
- Curetis NV (OpGen)
- DiaSorin S.p.A
- GenMark Diagnostics (Roche)
- Greiner Bio-One GmbH
- Lucira Health
- Meridian Bioscience, Inc.
- Mesa Biotech, Inc. (Thermo)
- QIAGEN NV
- QuantuMDx Group
- QuidelOrtho Corporation
- Roche
- Sekisui Diagnostics LLC
- T2 Biosystems
This report is an essential tool for understanding the size and growth opportunity in molecular POC. Molecular point of care (mPOC) has gone from novel trend to significant contributor to the market. The instruments sold by Abbott, BioMérieux, Cepheid and increasingly, a host of other companies, are a significant contributor to the IVD POC market. Knowing these markets is essential to knowing the opportunity in point of care testing and microbiology IVD.
Since 2013, Kalorama has been producing a dedicated report on systems and consumables. “The Market and Future Potential for Molecular Point of Care (POC), 2023” is Kalorama's latest. The report provides market sizing, forecasting, trend mapping and competitive analysis for point of care tests using PCR or other molecular technology with fast turnaround times and usability in near-patient settings.

Kalorama reports provide real-world analysis and model for relevant circumstances. As an example, analysts provide two different market perspectives. Near patient molecular testing using small instruments, and molecular point-of-care. Both markets are provided so the report can be useful for any business plans. As far as we know, Kalorama is the only market research resource providing this insight.
The data in “The Market and Future Potential for Molecular Point of Care (POC), 2023” includes information on systems and competitor analysis, as well as data on the size and growth of the market:
- What are the mPOC market opportunities beyond COVID-19?
- What are the current systems on the market? Who's winning?
- What's the size of the Molecular Point of Care Market: 2022-2027
- Geographic Breakout of the Molecular Point of Care Market, 2021 (N. America, Europe, APAC, RoW)
- Segment Breakout of Molecular Point of Care Market, 2022 (Respiratory vs. Other)
- Respiratory Segment Breakout, 2022 (Flu, RSV, Strep, Other)
- Breakout of mPOC Respiratory, 2022 (%; Flu, Strep, RSV, Other)
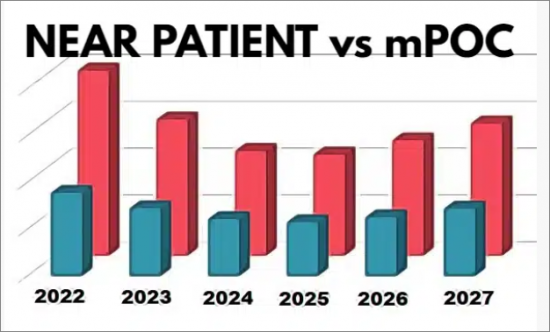
- Near Patient Molecular IVD Market: 2022-2027
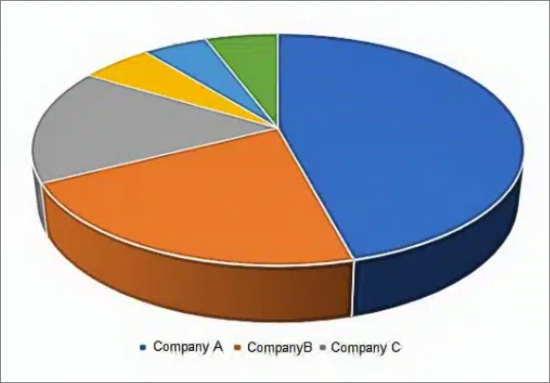
- Near Patient Molecular Market Share by Vendor, 2022
- Molecular Point of Care Market Share by Vendor, 2022
- Company Profiles
Many trends are covered in the report, including the role of COVID-19 testing, menu expansion, disease statistics, the COVID-19 crisis, immunoassay competition and enhancement of those competitive POC systems, emerging markets, new journal studies about the efficacy of mPOC, and other trends.
Market Share
The report contains market share for both the molecular and the near-term market

Companies covered in this report, in profiles and/or product tables and market developments discussion include:
|
|
Table of Contents
Chapter 1: Executive Summary
- Figure 1-1: Molecular Point of Care Market, 2022-2026 ($M)
- Figure 1-2: Near Patient Molecular Systems Market: 2022-2027 ($M)
- Where is Molecular Point of Care in 2023?
- Table 1-1: Molecular Point of Care Market, 2022-2027 ($M)
- Molecular Point of Care Market Analysis
- Table 1-2: Molecular Point of Care Market, 2022-2027 (%)
- Figure 1-3: mPOC Market by Disease Indication, 2022 (%; Respiratory, Other)
- Market Observations
- "Near-Patient" Molecular Systems Market
- Figure 1-4: mPOC and Near-Patient Market, 2022-2027
- Table 1-3: Near Patient Molecular Systems Market, 2022-2027 ($M and %)
- Current Trends
- Molecular Point-of-Care Diagnostics Defined
- Usage of Molecular Point of Care
- Leading mPOC Platforms
- Table 1-4: Molecular POC Diagnostic Platforms and Technologies
- Trends
- Scope and Methodology
Chapter 2: Molecular Point-of-Care Market Development and Trends
- COVID-19 and Molecular POC
- Table 2-1: Molecular POC COVID-19 Emergency Authorizations
- CDC Initial Response, Long Term Situation
- COVID-19's Unique Challenge
- BARDA Directs Funding to mPOC
- Recent Regulatory Developments
- Consortia, Funding, Prizes
- Deals
- Table 2-2: Deals in Molecular Point of Care, April 2019-January 2023
- Advantages and Disadvantages of Molecular Point of Care
- Table 2-3: Disadvantages of Molecular Point of Care, by Setting (Physician Office Laboratory (POL) / Other Outpatient Settings, Developing and Low-Resource Areas, and Hospital POC)
- Justification: The Sensitivity/Specificity Argument
- New Systems and Menu Expansion
- Mesa Biotech Strep A Approval
- Combination Tests Enter Market: SARS-CoV-2, Flu A, Flu B, and RSV
- STI Continues to Be a Growth Area
- Deals, Investment in mPOC Systems
- Thermo Acquires Mesa Biotech
- EU Researchers Awarded €3M to Develop POC Molecular Tests
- Scope Fluidics AST System Sees Investment
- China as a POC Market
- Common Tests and Analytes in POC Diagnostics
- Component Technologies of Molecular Point-of-Care Diagnostics
- Microfluidics
- qPCR
- Microarrays
- Isothermal Amplification
- Test Automation
- Primers and Probes
- Detection
- Next-Generation Sequencing
- Molecular Diagnostics
- Real-Time PCR (qPCR)
- Isothermal Amplification Methods
- Line Probe Assays
- Next-Generation Sequencing
- Applications and Potential Applications for Molecular Point-of-Care
- Major Testing Applications for Molecular POC Diagnostics
- Influenza
- Reclassification of RIDTs
- Hospital-Acquired Infections (HAIs)
- Strep A
- Respiratory Syncytial Virus (RSV)
- Emerging Applications
- Zika
- Other Respiratory Infections
- Group B Streptococcus
- Human Papillomavirus
- Herpes Simplex Virus
- Vaginitis
- Tuberculosis
- Malaria
- Other Tropical and Neglected Diseases
- Cancer
- European Device Regulations Nearing
Chapter 3: Market Analysis
- Molecular Point of Care Market Analysis
- Table 3-1: Molecular Point of Care Market, 2022-2027 ($M, %)
- Figure 3-1: Molecular Point of Care Market, 2022-2026 ($M)
- Table 3-2: Molecular Point of Care Market Share, by Vendor (Abbott, bioMérieux, Cepheid, Cue Health, Roche, Other), 2022 ($M, %)
- Figure 3-2: Molecular Point of Care Market Share, by Vendor (Abbott, bioMérieux, Cepheid, Cue Health, Roche, Other), 2022 (%)
- Table 3-3: Geographic Breakout of the Molecular Point of Care Market, 2022 (N. America, Europe, APAC, RoW)
- Table 3-4: Segment Breakout of Molecular Point of Care Market, 2022 (COVID-Respiratory vs. Other)
- Figure 3-3: Breakout of Molecular Point of Care Market (Respiratory, Other), 2022 (%)
- "Near Patient Molecular" Market
- Table 3-5: Near Patient Molecular Systems Market, 2022-2027
- Market Share Near Patient
- Figure 3-4: Market Share, Near Patient Molecular Testing, (Abbott, bioMérieux, Cepheid, Cue Health, Roche, Other), 2022
Chapter 4: Company Profiles
- Abbott Laboratories
- Aidian Oy
- Akonni Biosystems
- binx health, inc.
- Biocartis NV
- bioMerieux SA
- Table 4-1: bioMérieux FilmArray Est. Systems per Quarter, Q4 '18-Q3 '22
- Figure 4-1: FilmArray Systems Installed by Quarter, Q4 2018-Q3 2022
- Cepheid (Danaher)
- Molecular Healthcare-Acquired Infection (HAI) Testing
- Molecular Sexual Health and Women's Health Testing
- Molecular Critical Infectious Disease Testing
- Molecular Oncology/Genetics Testing
- Credo Diagnostics
- Cue Health
- Curetis NV (OpGen)
- DiaSorin S.p.A
- GenMark Diagnostics (Roche)
- Greiner Bio-One GmbH
- Lucira Health
- Meridian Bioscience, Inc.
- Mesa Biotech, Inc. (Thermo)
- QIAGEN NV
- QuantuMDx Group
- QuidelOrtho Corporation
- Roche
- Sekisui Diagnostics LLC
- T2 Biosystems













