 |
市場調查報告書
商品編碼
1402601
血管閉合裝置市場:依產品類型、通路、孔尺寸、最終用戶、地區Vascular Closure Device Market, by Product Type, By Access, By Hole Size By End User and by Region |
||||||
血管閉合裝置的全球市場規模預計將從 2023 年的 12.838 億美元增加到 2030 年的 19.432 億美元,預測期內複合年成長率為 6.1%。
| 報告範圍 | 報告詳情 | ||
|---|---|---|---|
| 基準年 | 2022年 | 2023年市場規模 | 12.838 億美元 |
| 實際資料 | 2018-2021 | 預測期 | 2023-2030 |
| 預測期複合年成長率 | 6.10% | 2030年市場規模預測 | 19.432 億美元 |
血管閉合裝置有助於在血管造影術或血管成形術等心血管手術後密封動脈穿孔。它透過機械或生物手段促進自然凝血,有助於穿刺部位止血。隨著全球介入性心臟病學和放射治療的發展,血管閉合裝置的全球使用量不斷增加。與手動壓迫方法相比,血管閉合裝置可以改善患者舒適度、縮短住院時間並降低併發症率,這使得血管閉合裝置對於血管通路部位的管理至關重要。
市場動態:
由於人口變化和技術進步等多種因素,全球血管閉合裝置市場正經歷顯著成長。造成這種成長的一個主要因素是老年人口的增加,由於生理衰老,他們更容易患心血管疾病。隨著老年人口的增加,心血管疾病的發生率也隨之增加,導致診斷和治療程序後對有效血管閉合裝置的需求增加。同時,整個醫療保健產業向微創手術 (MIS) 的轉變已經變得越來越明顯。與傳統開放性手術相比,MIS手術可降低感染風險、縮短住院時間並加速患者復原速度。血管閉合裝置在這些手術中發揮重要作用,包括快速止血、減少行走時間和提高患者舒適度。
儀器技術的進步也至關重要,它可以使儀器更有效並降低術後併發症的風險。設計和材料的改進使得產品能夠提供更好的密封性並與各種解剖形狀和穿刺尺寸相容。另一方面,與這些先進設備相關的高成本可能會成為普及的障礙。某些醫療保健系統缺乏報銷進一步加劇了這個問題,使患者難以獲得這些技術。現有的替代方法可以實現止血,例如手動壓迫,但它們通常會導致恢復時間更長並增加併發症的風險。
這個市場提供了龐大的商機,特別是在目前正在加強醫療保健和醫療基礎設施投資的新興國家。新型設備的持續開發具有巨大潛力,例如由生物分解性材料製成的設備,通常用於經導管主動脈瓣置換術術 (TAVR) 等複雜手術。總體而言,儘管成本和報銷問題等挑戰仍然存在,但血管閉合裝置的優勢,例如技術進步和對 MIS 需求的增加,可能會繼續推動市場成長。
例如,2021年1月,專注於提供創新醫療保健解決方案以促進改善患者治療效果的醫療技術公司Haemonetics Corporation達成了一項最終協定,收購了血管閉合系統製造商 Cardiva Medical, Inc.。
本研究的主要特點
- 該報告對全球血管閉合器械市場進行了詳細分析,並提供了以2022年為基準年的預測期(2023-2030)的市場規模和年複合成長率(CAGR%)。
- 它還揭示了各個細分市場的潛在商機,並為該市場說明了一系列有吸引力的投資提案。
- 它還提供了有關市場促進因素、抑制因素、機會、新產品發布和核准、市場趨勢、區域前景、主要企業採取的競爭策略等的主要考察。
- 它根據公司亮點、產品系列、主要亮點、績效和策略等參數,介紹了全球血管閉合裝置市場的主要企業。
- 該報告的見解使行銷人員和經營團隊負責人就未來的產品發布、類型升級、市場擴張和行銷策略做出明智的決策。
- 全球血管閉合裝置市場報告滿足該行業的各種相關人員,例如投資者、供應商、產品製造商、經銷商、新進入者和財務分析師。
- 透過用於分析全球血管閉合裝置市場的各種策略矩陣,將促進相關人員的決策。
目錄
第1章 研究目的與前提
- 研究目標
- 先決條件
- 簡稱
第2章 市場展望
- 報告說明
- 市場定義和範圍
- 執行摘要
- Coherent Opportunity Map(COM)
第3章市場動態、法規及趨勢分析
- 市場動態
- 老年人口增加
- 血管成形術迅速增加
- 心血管疾病盛行率增加
- 微創手術需求增加
- 影響分析
- 主要亮點
- 監管場景
- 產品發布/核准
- PEST分析
- 波特的分析
- 併購場景
第4章全球血管閉合裝置市場 - 冠狀冠狀病毒(COVID-19) 大流行的影響
- 新型冠狀病毒感染疾病(COVID-19)的流行病學
- 供需面分析
- 經濟影響
第5章全球血管閉合裝置市場,依產品類型,2023-2030
- 被動血管閉合裝置
- 主動血管閉合裝置
- 體外止血裝置
第6章 全球血管閉合裝置市場,Access,2023-2030
- 股骨
- 徑向
第 7 章 全球血管閉合裝置市場(按孔尺寸),2023-2030 年
- 小孔
- 長孔
第 8 章全球血管閉合裝置市場,依最終用戶分類,2023-2030 年
- 醫院
- 門診手術中心
- 其他
第9章全球血管閉合裝置市場,按地區,2023-2030
- 北美洲
- 美國
- 加拿大
- 歐洲
- 德國
- 英國
- 西班牙
- 法國
- 義大利
- 俄羅斯
- 其他歐洲國家
- 亞太地區
- 中國
- 印度
- 日本
- 澳洲
- 韓國
- ASEAN
- 其他亞太地區
- 拉丁美洲
- 巴西
- 阿根廷
- 墨西哥
- 其他拉丁美洲
- 中東
- 海灣合作理事會國家
- 以色列
- 其他中東地區
- 非洲
- 南非
- 北非
- 中部非洲
第10章競爭形勢
- Abbott
- Cardiva Medical Inc.
- Terumo Corporation
- Johnson & Johnson Services Inc.
- Braun SE
- Biotronik SE & Co. KG
- MicroPort Scientific Corporation
- ConforMIS, Inc.
- Medtronic
- Transluminal Technologies LLC
- Cardinal Health
- Teleflex Incorporated
- Vasorum Ltd.
- Tricol Biomedical
- Merit Medical Systems, Inc.
- Stryker
第11章 章節
- 調查方法
- 關於出版商
The global vascular closure device market size is expected to reach US$ 1,943.2 Mn by 2030, from US$ 1,283.8 Mn in 2023, at a CAGR of 6.1% during the forecast period.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2022 | Market Size in 2023: | US$ 1,283.8 Mn |
| Historical Data for: | 2018 to 2021 | Forecast Period: | 2023 - 2030 |
| Forecast Period 2023 to 2030 CAGR: | 6.10% | 2030 Value Projection: | US$ 1,943.2 Mn |

Vascular closure devices help seal arterial punctures after a cardiovascular procedure such as angiography or angioplasty. They help stop bleeding at the puncture site by accelerating natural clotting through mechanical or biological means. The global usage of vascular closure devices has been increasing with growth in interventional cardiology and radiology procedures worldwide. Improved patient comfort, reduced hospital stay times, and lower complication rates compared to manual compression methods have made vascular closure devices an integral part of vascular access site management.
Market Dynamics:
The global vascular closure device market is growing substantially, propelled by a confluence of factors ranging from demographic shifts to technological progress. A significant contributor to this growth is the rising geriatric population which is more susceptible to cardiovascular diseases due to physiological aging. As the elderly population grows, so does the incidence of cardiovascular conditions, thereby increasing the demand for effective vascular closure devices following diagnostic and therapeutic procedures. Simultaneously, a notable shift towards minimally invasive surgeries (MIS) across the healthcare sector. MIS procedures reduce the risk of infection, shorten hospital stays, and speed up patient recovery times compared to traditional open surgeries. Vascular closure devices play a key role in these procedures by providing rapid hemostasis, reducing time to ambulation, and enhancing patient comfort.
Advancements in device technology have also been pivotal, leading to devices that offer greater efficacy and a reduced risk of post-procedure complications. Enhanced designs and materials have yielded products with improved sealing capabilities and compatibility with various anatomies and puncture sizes. On the other side of the spectrum, the high costs associated with these advanced devices can act as a barrier to their widespread adoption. The lack of reimbursement in certain healthcare systems further exacerbates this issue, making it challenging for patients to access these technologies. While existing alternatives for achieving hemostasis, such as manual compression, exist, they often come with longer recovery times and elevated risks of complications, thereby providing an opening for the adoption of advanced vascular closure devices.
The market is ripe with opportunities, especially within emerging economies that are currently witnessing increased healthcare investments and enhancements to their healthcare infrastructure. The significant potential in the ongoing development of novel devices, including those made from biodegradable materials, commonly used in complex procedures like transcatheter aortic valve replacement (TAVR).Overall, while challenges like cost and reimbursement issues persist, the benefits of vascular closure devices, including the advancements in technology and rising demands for MIS, are likely to continue to drive market growth.
For instance, in January 2021, Haemonetics Corporation, a medical technology company focused on delivering innovative medical solutions to drive better patient outcomes, entered into a definitive agreement to acquire privately-held Cardiva Medical, Inc., a manufacturer of vascular closure systems.
Key features of the study:
- This report provides in-depth analysis of the global vascular closure device market, and provides market size (US$ Mn) and compound annual growth rate (CAGR%) for the forecast period (2023-2030), considering 2022 as the base year
- It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market
- This study also provides key insights about market drivers, restraints, opportunities, new product launches or approval, market trends, regional outlook, and competitive strategies adopted by key players
- It profiles key players in the global vascular closure device market based on the following parameters - company highlights, products portfolio, key highlights, financial performance, and strategies
- Key companies covered as a part of this study include Abbott, Cardiva Medical Inc., Terumo Corporation, Johnson & Johnson Services Inc., B. Braun SE, Biotronik SE & Co. KG, MicroPort Scientific Corporation, ConforMIS, Inc., Medtronic, Transluminal Technologies LLC, Cardinal Health, Teleflex Incorporated, Vasorum Ltd., Tricol Biomedical, Merit Medical Systems, Inc., Stryker
- Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics
- The global vascular closure device market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts
- Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the global vascular closure device market
Detailed Segmentation:
Global Vascular Closure Device Market
- By Product Type
- Passive Vascular Closure Devices
- Active Vascular Closure Devices
- External Hemostatic Devices
- By Access
- Femoral
- Radial
- By Hole Size
- Small Hole
- Large Hole
- By End User
- Hospitals
- Ambulatory Surgery Centers
- Others
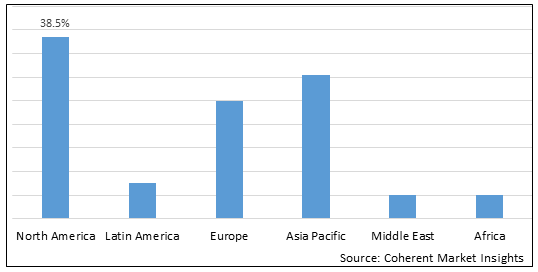
- By Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East
- Africa
- Top companies in Global Vascular Closure Device Market:
- Abbott
- Cardiva Medical Inc.
- Terumo Corporation
- Johnson & Johnson Services Inc.
- B.Braun SE
- Biotronik SE & Co. KG
- MicroPort Scientific Corporation
- ConforMIS, Inc.
- Medtronic
- Transluminal Technologies LLC
- Cardinal Health
- Teleflex Incorporated
- Vasorum Ltd.
- Tricol Biomedical
- Merit Medical Systems, Inc.
- Stryker
Table of Contents
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- Vascular Closure Device Market, By Product Type
- Vascular Closure Device Market, By Access
- Vascular Closure Device Market, By Hole Size
- Vascular Closure Device Market, By End User
- Vascular Closure Device Market, By Region
- Coherent Opportunity Map (COM)
3. Market Dynamics, Regulations, and Trends Analysis
- Market Dynamics
- Driver
- Rising geriatric population
- The proliferation of angioplasty procedures
- The increasing prevalence of cardiovascular diseases incidence
- Increasing demand for minimally invasive surgeries
- Impact Analysis
- Key Highlights
- Regulatory Scenario
- Product launch/Approvals
- PEST Analysis
- PORTER's Analysis
- Merger and Acquisition Scenario
4. Global Vascular Closure Device Market- Impact of Coronavirus (COVID-19) Pandemic
- COVID-19 Epidemiology
- Supply Side and Demand Side Analysis
- Economic Impact
5. Global Vascular Closure Device Market, By Product Type, 2023-2030, (US$ Mn)
- Introduction
- Market Share Analysis, 2023 and 2030 (%)
- Y-o-Y Growth Analysis, 2019 - 2030
- Segment Trends
- Passive Vascular Closure Devices
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030,(US$ Mn)
- Active Vascular Closure Devices
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030,(US$ Mn)
- External Hemostatic Devices
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030,(US$ Mn)
6. Global Vascular Closure Device Market, By Access, 2023-2030, (US$ Mn)
- Introduction
- Market Share Analysis, 2023 and 2030 (%)
- Y-o-Y Growth Analysis, 2019 - 2030
- Segment Trends
- Femoral
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030,(US$ Mn)
- Radial
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030,(US$ Mn)
7. Global Vascular Closure Device Market, By Hole Size, 2023-2030, (US$ Mn)
- Introduction
- Market Share Analysis, 2023 and 2030 (%)
- Y-o-Y Growth Analysis, 2019 - 2030
- Segment Trends
- Small Hole
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030,(US$ Mn)
- Large Hole
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030,(US$ Mn)
8. Global Vascular Closure Device Market, By End User, 2023-2030, (US$ Mn)
- Introduction
- Market Share Analysis, 2023 and 2030 (%)
- Y-o-Y Growth Analysis, 2019 - 2030
- Segment Trends
- Hospitals
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030,(US$ Mn)
- Ambulatory Surgery Centers
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030,(US$ Mn)
- Others
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2018-2030,(US$ Mn)
9. Global Vascular Closure Device Market, By Region, 2023-2030, (US$ Mn)
- Introduction
- Market Share Analysis, By Country, 2023 and 2030 (%)
- Y-o-Y Growth Analysis, For Country 2019 -2030
- Country Trends
- North America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Product Type, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Access, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Hole Size, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2018-2030,(US$ Mn)
- U.S.
- Canada
- Europe
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Product Type, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Access, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Hole Size, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2018-2030,(US$ Mn)
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Product Type, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Access, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Hole Size, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2018-2030,(US$ Mn)
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Latin America
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Product Type, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Access, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Hole Size, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2018-2030,(US$ Mn)
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Middle East
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Product Type, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Access, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Hole Size, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Country, 2018-2030,(US$ Mn)
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, By Product Type, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Access, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Hole Size, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By End User, 2018-2030,(US$ Mn)
- Market Size and Forecast, and Y-o-Y Growth, By Country/Region, 2018-2030,(US$ Mn)
- South Africa
- North Africa
- Central Africa
10. Competitive Landscape
- Abbott
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Cardiva Medical Inc.
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Terumo Corporation
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Johnson & Johnson Services Inc.
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Braun SE
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Biotronik SE & Co. KG
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- MicroPort Scientific Corporation
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- ConforMIS, Inc.
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Medtronic
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Transluminal Technologies LLC
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Cardinal Health
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Teleflex Incorporated
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Vasorum Ltd.
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Tricol Biomedical
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Merit Medical Systems, Inc.
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Stryker
- Company Highlights
- Product Portfolio
- Key Highlights
- Financial Performance
- Strategies
- Analyst Views
11. Section
- Research Methodology
- About us












