![頸動脈疾病(CAD)市場:1次調查(KOL的洞察) - 市場情報 - 流行病學和到2033年前的市場預測 [CEA,CAS,TCAR手藝市場]](https://www.gii.tw/sample/img/cover/75/1307858.png) |
市場調查報告書
商品編碼
1307858
頸動脈疾病(CAD)市場:1次調查(KOL的洞察) - 市場情報 - 流行病學和到2033年前的市場預測 [CEA,CAS,TCAR手藝市場]Carotid Artery Disease (CAD)| Primary Research (KOL's Insight) | Market Intelligence | Epidemiology & Market Forecast-2033 [CEA, CAS & TCAR Procedure Market] |
||||||
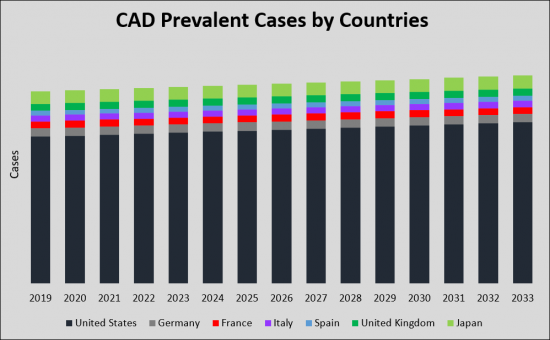
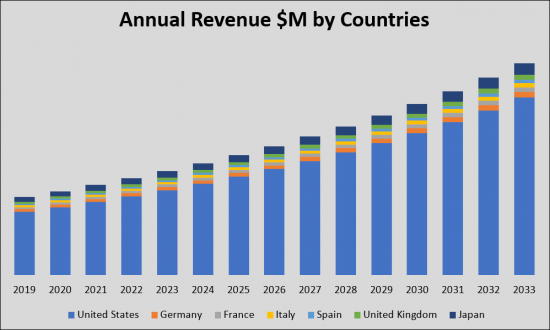
由於頸動脈疾病(CAD)患病率不斷上升以及對更有效、更方便的治療方案的需求,預計頸動脈疾病(CAD)市場將顯著增長。由於流行率、市場規模和創新療法的推出預計將增加,CAD 市場為製藥公司和醫療保健組織提供了利潤豐厚的機會。美國、五個歐洲國家和日本七國集團國家目前主導著CAD治療市場。到2033年,這些國家的市場規模預計將從2019年的24億美元大幅增長,複合年增長率穩定。2019年,美國市場佔有率最大,為19億美元,其次是歐洲五個國家,市場佔有率為3億美元,日本為1.5億美元。由於新療法的出現,預計到 2033 年整體市場將擴大。
本報告提供全球頸動脈疾病(CAD)市場相關調查,提供市場現狀,以及病例數趨勢,患者趨勢,競爭產品的市場上地位,市場機會等資訊。
目錄
摘要整理
頸動脈疾病的背景
頸動脈疾病診斷
- 頸動脈疾病(CAD):診斷概要
- 2021年血管外科學會(SVS)診斷指南
流行病學和患者族群
- 主要調查結果
- 方法和資料來源
頸動脈疾病的流行病學和模式參數的主要的資訊來源
- 美國
- 德國
- 法國
- 義大利
- 西班牙
- 英國
- 日本
目前醫療行為
已上市設備
- 已上市及新興支架概要
- Acculink(雅培)
- Xact(雅培)
- PRECISE PRO RX 鎳鈦合金支架系統 (Cordis)
- Protege RX(美敦力)
- CGuardEPS(啟發醫學博士)
- Neuroguard IEP(Contego Medical)
- 頸動脈壁支架(波士頓科學)
- ENROUTE 頸動脈支架系統(絲路醫療)
- MER(巴爾頓)
- Casper/Roadsaver(Microvention-Terumo, Inc.)
未滿足需求
頸動脈疾病領域的開發平台(管線)開發
- 產品分析
- CGuard Prime(Inspire MD)
- 短軸 CGuard (Inspire MD)
頸動脈疾病(CAD)- 定價與給付的研究
頸動脈疾病(CAD)- 競爭上的地位
市場預測
- 主要調查結果
- 概要
- 到2033年前的各國市場預測
各國市場預測
- 美國
- 德國
- 法國
- 義大利
- 西班牙
- 英國
- 日本
推動市場要素與阻礙因素
附錄
The Carotid Artery Disease (CAD) market is undergoing substantial growth and transformation, driven by the increasing prevalence of CAD and the need for more effective and convenient treatment options. With the projected rise in prevalent cases and market size, as well as the introduction of innovative therapies, the CAD market presents lucrative opportunities for pharmaceutical companies and healthcare providers. In terms of geographical distribution, the G7 countries-United States, European five countries, and Japan-currently dominate the CAD therapy market. By 2033, the market size in these countries will significantly increase from USD 2.4 billion in 2019, with a steady CAGR. In 2019, the United States held the largest market share at USD 1.9 billion, followed by the European five countries at USD 0.3 billion and Japan at USD 0.15 billion. With the advent of new therapies, the overall market is expected to expand by 2033.
"Personally speaking, I think that it is absolutely essential that we identify criteria for defining patients who are at high risk for stroke with an asymptomatic carotid stenosis in whom we should target CEA or CAS. In the US, over 90% of carotid interventions are performed in asymptomatic patients (120,000 per year). However, if we assume that the 1995 ACAS (Asymptomatic Carotid Atherosclerosis Study) data still have any relevance in 2013 (although it probably does not), 95% of all carotid interventions in asymptomatic patients are ultimately unnecessary. Even if the procedural risk of CEA and CAS could be reduced to zero, 93% of all interventions would still be unnecessary."
Carotid stenosis/ carotid artery disease (CAD) is caused by a buildup of plaque (atherosclerosis) inside the artery wall that reduces blood flow to the brain. Older people are more likely to be affected by carotid stenosis. Before age 75, men are more at risk than women. A person who has high cholesterol, has high blood pressure, and smokes is eight times more likely to develop atherosclerosis than a person without these risk factors. Carotid artery disease develops slowly. If it increases to the point that a carotid artery is blocked, or blood flow is otherwise seriously reduced, a stroke can occur.
The primary goal in treating carotid artery disease is to prevent stroke. The choice of treatment depends on the degree of blockage in the carotid arteries, whether the blockage is causing symptoms, and the age and overall health of the individual. For those with mild to moderate blockage, lifestyle changes to slow the accumulation of fatty deposits and medications to control blood pressure or lower cholesterol are often recommended. In cases of severe blockage or for individuals who have experienced a Transient Ischemic Attack (TIA) or stroke, removal of the blockage might be necessary. The most common procedures are Carotid Endarterectomy (CEA) and Carotid Angioplasty and Stenting (CAS). Many patients with asymptomatic CAD do not receive any medical treatment.
Carotid Artery Disease (CAD) -Epidemiology
The total prevalent cases of Carotid Artery Disease (CAD) in the G7 countries are projected to increase from 11.45 million in 2019 to 2033 with a CAGR of 0.6% for the study period (2019-2033 ). According to estimates, the United States accounted for the highest incidence of Carotid Artery Disease (CAD) cases during the study period. Among the EU5, Germany had the highest cases of Carotid Artery Disease (CAD), followed by UK, France, Italy, and Spain. Japan is reported to have the highest number of cases after the United States.
Procedural Insights Carotid endarterectomy (CEA) and carotid artery stenting (CAS) are well-established treatments for CAD. However, their usage varies by region, with CEA being the preferred option in most areas due to its longer track record and proven efficacy. The introduction of newer procedures like Transcarotid Artery Revascularization (TCAR) has added to the treatment landscape, although its adoption is currently limited. Based on insights from Key Opinion Leaders (KOLs), diagnosis and treatment rates are expected to improve with increased awareness and access to better diagnostic tools and treatment options.

Carotid Artery Disease (CAD) - Current Market Size & Forecast Trends
The G7 therapeutic market for Carotid Artery Disease (CAD) is on a trajectory of significant growth from 2019 to 2033. Driven by an increase in the application and enhancement of existing treatment methods such as Carotid Endarterectomy (CEA), Carotid Artery Stenting (CAS), and Transcarotid Artery Revascularization (TCAR) and the launch of new products, this market is expected to expand from USD 2.4 billion in 2019. Emerging devices are expected to be launched in the Carotid Artery Disease CAS and TCAR space and will contribute to the increase in devices market share in the CAS and TCAR setting. Specifically, Inspire MD is introducing their second-generation devices for CAS, the CGuard Prime, and for TCAR, the Short Shaft CGuard, which are expected to further contribute to the overall market's growth.
The use of procedures like Carotid Endarterectomy (CEA), Carotid Artery Stenting (CAS), and Transcarotid Artery Revascularization (TCAR) to treat Carotid Artery Disease (CAD) is expected to increase significantly in the coming years. The reasons for this growth include an aging population, which leads to a higher prevalence of CAD, technological advances that make these procedures safer and more efficient, improved patient outcomes due to better procedural techniques and patient selection, higher healthcare expenditure by nations, and greater awareness leading to early detection of CAD.
The CAD market is further split by devices/procedures, with the Carotid Endarterectomy (CEA), Carotid Artery Stenting (CAS), and Transcarotid Artery Revascularization (TCAR) markets expected to experience significant growth by 2033, reflecting substantial market expansion.

Report Highlights:
- Carotid Artery Disease (CAD) - Current Market Trends
- Carotid Artery Disease (CAD) - Market Opportunities and Sales Potential for Agents
- Carotid Artery Disease (CAD) - Patient-based Market Forecast to 2033
- Carotid Artery Disease (CAD) - Untapped Business Opportunities
- Carotid Artery Disease (CAD) - Product Positioning Vis-a-vis Competitors' Products
- Carotid Artery Disease (CAD) - KOLs Insight
Table of Contents
Executive Summary
- Key Findings
Carotid Artery Disease Background
- Carotid Artery Disease Definition
- Overview Flow Diagram
- Causes
- Risk Factors
- Signs & Symptoms
- Pathophysiology
- Staging
Carotid Artery Disease Diagnosis
- Carotid Artery Disease (CAD): Diagnosis Overview
- 2021 Society for Vascular Surgery (SVS) Diagnostic Guidelines
Epidemiology and Patient Populations
- Key Findings
- Methods and data Sources
- Total G7 Prevalent cases of Carotid Artery Disease
- Total G7 Diagnosed, Treated, and Deemed High Risk Cases of Carotid Artery Disease
- Cases by procedure Type [carotid endarterectomy (CEA), carotid artery stenting (CAS), and transcarotid artery revascularization (TCAR)]
Key Sources for Carotid Artery Disease Epidemiology and Model Parameters
- United States
- United States Prevalent cases of Carotid Artery Disease
- United States Diagnosed, Treated, and Deemed High Risk Cases of Carotid Artery Disease
- Cases by procedure Type [carotid endarterectomy (CEA), carotid artery stenting (CAS), and transcarotid artery revascularization (TCAR)]
- Germany
- Germany Prevalent cases of Carotid Artery Disease
- Germany Diagnosed, Treated, and Deemed High Risk Cases of Carotid Artery Disease
- Cases by procedure Type [carotid endarterectomy (CEA), carotid artery stenting (CAS), and transcarotid artery revascularization (TCAR)]
- France
- France Prevalent cases of Carotid Artery Disease
- France Diagnosed, Treated, and Deemed High Risk Cases of Carotid Artery Disease
- Cases by procedure Type [carotid endarterectomy (CEA), carotid artery stenting (CAS), and transcarotid artery revascularization (TCAR)]
- Italy
- Italy Prevalent cases of Carotid Artery Disease
- Italy Diagnosed, Treated, and Deemed High Risk Cases of Carotid Artery Disease
- Cases by procedure Type [carotid endarterectomy (CEA), carotid artery stenting (CAS), and transcarotid artery revascularization (TCAR)]
- Spain
- Spain Prevalent cases of Carotid Artery Disease
- Spain Diagnosed, Treated, and Deemed High Risk Cases of Carotid Artery Disease
- Cases by procedure Type [carotid endarterectomy (CEA), carotid artery stenting (CAS), and transcarotid artery revascularization (TCAR)]
- United Kingdom
- United Kingdom Prevalent cases of Carotid Artery Disease
- United Kingdom Diagnosed, Treated, and Deemed High Risk Cases of Carotid Artery Disease
- Cases by procedure Type [carotid endarterectomy (CEA), carotid artery stenting (CAS), and transcarotid artery revascularization (TCAR)]
- Japan
- Japan Prevalent cases of Carotid Artery Disease
- Japan Diagnosed, Treated, and Deemed High Risk Cases of Carotid Artery Disease
- Cases by procedure Type [carotid endarterectomy (CEA), carotid artery stenting (CAS), and transcarotid artery revascularization (TCAR)]
Current Medical Practice
- Carotid Revascularization Devices Explanations: CEA, CAS & TCAR
- Guidelines
- 2021 Society for Vascular Surgery (SVS) clinical practice guidelines
- European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines
- Key Difference between SVS and ESVS Treatment Guidelines
- 2021 Japan Stroke Society Guideline
Marketed Devices Chapters
- Summary of Marketed & Emerging Stents
- Acculink (Abbott)
- Product Profile
- Clinical Profile
- Sales & Forecast to 2033
- Xact (Abbott)
- Product Profile
- Clinical Profile
- Sales & Forecast to 2033
- PRECISE PRO RX Nitinol Stent System (Cordis)
- Product Profile
- Clinical Profile
- Sales & Forecast to 2033
- Protégé RX (Medtronic)
- Product Profile
- Clinical Profile
- Sales & Forecast to 2033
- CGuardEPS (Inspire MD)
- Product Profile
- Clinical Profile
- Sales & Forecast to 2033
- Neuroguard IEP (Contego Medical)
- Product Profile
- Clinical Profile
- Sales & Forecast to 2033
- Carotid WALLSTENT (Boston Scientific)
- Product Profile
- Clinical Profile
- Sales & Forecast to 2033
- ENROUTE Transcarotid Stent System (SilkRoad Medical)
- Product Profile
- Clinical Profile
- Sales & Forecast to 2033
- MER (Balton)
- Product Profile
- Clinical Profile
- Sales & Forecast to 2033
- Casper/Roadsaver (Microvention-Terumo, Inc.)
- Product Profile
- Clinical Profile
- Sales & Forecast to 2033
Unmet Needs
Pipeline Developments in the Carotid Artery Disease space
- Product Analysis
- CGuard Prime (Inspire MD)
- Product Profile
- Clinical Development
- Market & Sales Opportunity Forecasted to 2033
- Short Shaft CGuard (Inspire MD)
- Product Profile
- Clinical Development
- Market & Sales Opportunity Forecasted to 2033
- CGuard Prime (Inspire MD)
Carotid Artery Disease (CAD)- Pricing and Reimbursement Studies
- Fixed and variable cost of carotid endarterectomy and stenting in the United States: A comparative study
- Comparative Analysis of the estimated cost profiles of TCAR, TF-CAS, and CEA
Carotid Artery Disease (CAD)- Competitive Positioning
- Future Treatment Paradigm
- Carotid Artery Disease (CAD) Competitor Landscape and Approvals Anticipated
- Annual Cost of CEA, CAS and TCAR procedures
Market Outlook
- Key Findings
- Overview
- Country Specific Market Forecast to 2033
- Total Market of Carotid Artery Disease (2019-2033)
- Market Share of Carotid Artery Disease by Surgical Procedures (2019-2033)
- Market Share of Carotid Artery Disease by Companies and CAS & TCAR Devices (2019-2033)
- Total Market of Carotid Artery Disease (2019-2033)
- Market Share of Carotid Artery Disease by Company for carotid endarterectomy (CEA) Procedures (2019-2033)
- Market Share of Carotid Artery Disease by Company for carotid artery stenting (CAS) Procedures (2019-2033)
- Market Share of Carotid Artery Disease by Company for carotid endarterectomy (CEA) Procedures (2019-2033)
- Total Market of Carotid Artery Disease (2019-2033)
Market Forecast by Country
- United States
- Total Market of Carotid Artery Disease (2019-2033)
- Market Share of Carotid Artery Disease by Companies and CAS & TCAR Devices (2019-2033)
- Germany
- Total Market of Carotid Artery Disease (2019-2033)
- Market Share of Carotid Artery Disease by Companies and CAS & TCAR Devices (2019-2033)
- France
- Total Market of Carotid Artery Disease (2019-2033)
- Market Share of Carotid Artery Disease by Surgical Procedures (2019-2033)
- Market Share of Carotid Artery Disease by Companies (2019-2033)
- Italy
- Total Market of Carotid Artery Disease (2019-2033)
- Market Share of Carotid Artery Disease by Surgical Procedures (2019-2033)
- Market Share of Carotid Artery Disease by Companies (2019-2033)
- Spain
- Total Market of Carotid Artery Disease (2019-2033)
- Market Share of Carotid Artery Disease by Surgical Procedures (2019-2033)
- Market Share of Carotid Artery Disease by Companies (2019-2033)
- United Kingdom
- Total Market of Carotid Artery Disease (2019-2033)
- Market Share of Carotid Artery Disease by Surgical Procedures (2019-2033)
- Market Share of Carotid Artery Disease by Companies (2019-2033)
- Japan
- Total Market of Carotid Artery Disease (2019-2033)
- Market Share of Carotid Artery Disease by Surgical Procedures (2019-2033)
- Market Share of Carotid Artery Disease by Companies (2019-2033)
Market Drivers and Constraints
- What Factors Are Driving the Market for Carotid Artery Disease?
- What Factors Are Constraining the Market for Carotid Artery Disease?
Appendix
- Methodology
- 2020 AWMF Guidelines: Diagnostic Recommendations
- 2023 ESVS European Treatment Guidelines





