 |
市場調查報告書
商品編碼
1170397
抗C-C動機趨化素受體8(CCR8)-開發平台分析:2022年Anti-C-C motif chemokine receptor 8 (CCR8)-Pipeline Analytics 2022 |
||||||
本報告提供抗CCR8市場機會的主要的競爭分析,開發平台醫藥品的簡介的企業,臨床試驗,其他的發展趨勢等彙整資料。
樣本圖
目錄
概要
抗CCR8標的背景
- CC動機趨化素受體8(CCR8)- 概要
- 治療標的的CCR8
- CCR8臨床性展望
- CCR8標靶治療相關的安全上的疑慮
抗CCR8開發平台分析,各相
- 抗CCR8開發- 概要
- 開發中產品,各開發階段
- 抗CCR8競爭情形環境,各相
- 開發中產品,各企業
- 開發中產品,適應症及各相
- 抗CCR8臨床及法規的單劑療法及聯合治療
- 抗CCR8資產,適應症及各相
- 抗CCR8臨床及法規的時間軸
抗CCR8授權,收購,及合作契約
抗形勢開發平台狀況
- 簡介比較概要
- 抗CCR8開發平台的藥物簡介
- 第一/二階段
- BMS-986340
- S-531011
- LM-108
- 第一階段
- ICP B05
- BAY 3375968
- GS 1811
- IPG 7236
- ABBV 514
- 前臨床
- FPA157
- HBM1022
- CCR8 Treg計劃
- ZL-1218
- SRF 114
- BCG 005
- GB2101
- FG-3136
- CHS-3318
- 藥物研發
- 抗CCR8抗體
- 第一/二階段
抗CCR8 SWOT分析
抗CCR8產品的定位
附錄
The Anti-CCR8 report covers the anti-CCR8 market opportunity providing Key Competitive Analysis, 18+ Companies with Pipeline Drug Profiles, Clinical Trials, Other Developments (Collaboration Details, Funding, etc.), Licensing and Agreements, Business Agreements, Business Partners as well as Clinical Partner. The report covers pipeline product analysis by stage of development, competitive landscape by phases, companies, therapy area, and indication by phases. The Anti-CCR8 report adds value in terms of describing clinical-stage products concerning their clinical & regulatory timelines as well as in terms of providing the current market opportunity, drivers, and challenges.
SAMPLE VIEW

The Anti-CCR8-mediated Treg depletion specifically in tumor tissues for a limited period could avoid deleterious autoimmunity and immunopathology in mice, despite CCR8 expression by some tissue-resident Tregs (46). This lack of immunological adverse effects can be attributed not only to the selective depletion of tumor-reactive Tregs by anti-CCR8 mAb but also to the differences in the antigenicity of normal self-antigens versus quasi-self-tumor antigens, and the differences between the tumor microenvironment and the normal tissue.
In this report, Mellalta Meets provides an in-depth analysis of Anti-CCR8 covering discovery, preclinical and clinical studies, details of partnerships and business deal values, targeted technologies and therapy areas, investments, and acquisition trends. Currently, there are more than 17 candidate Anti-CCR8 products currently under evaluation in clinical and preclinical studies. The major key players operating in the market are Bristol-Myers Squibb, Shionogi, LaNova Medicines, InnoCare Pharma/Keymed Biosciences, Bayer, and many more which have robust clinical pipelines of Anti-CCR8 candidates.
As per analysis, the development pipeline is full of molecules like small molecules, antibodies, and combination therapies.
Key Highlights of the Anti-CCR8 Report:
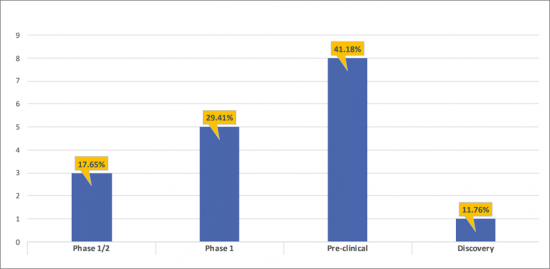
- There are ~8 products in the Pre-clinical stage of development, and ~5 in the Phase 1 stage of development representing 41.18%, and 29.41% respectively of the total share of the developing Anti-CCR8 landscape. The remaining 29.41% has been contributed by Phase 1/2 (17.65%), and Discovery (11.76%) assets.
- The pipeline of Anti-CCR8 is dominated by biotech companies headquartered in China with Chinese companies holding the top 7 positions. They represent ~38.89% of all the pipeline Anti-CCR8 in clinical stages.
- Anti-CCR8 pipeline landscape includes 47% of Monotherapy trials and 53% of Combination Trials
- The pipeline of Anti-CCR8 is dominated by pre-clinical (8); Phase 1 (5); Phase 1/2 (3) assets and Discovery (1) assets.
- To be continued....
Report Coverage:
- Indication Prioritisation: Anti-CCR8 market potential based on Indications
- Business Transactions & Strategies: Key collaborations and deal values
- Anti-CCR8 Pipeline Development: Product Profiles, Clinical Trials & Results
- Anti-CCR8 Acquisition Targets
- Anti-CCR8 Competitive Intelligence
- Recent & Upcoming events
TABLE OF CONTENTS
OVERVIEW
The Anti-CCR8 Target BACKGROUND
- C-C Motif Chemokine Receptor 8 (CCR8)-Overview
- CCR8 as a Therapeutic Target
- Clinical Prospects of CCR8
- Safety Concerns Related to CCR8-Targeted Therapies
Anti-CCR8 PIPELINE ANALYSIS by Phases
- Anti-CCR8 Development - Overview
- Pipeline Products by Stage of Development
- Anti-CCR8 Competitive Landscape by Phases
- Pipeline Products by Company
- Pipeline Products by Indication and Phases
- Anti-CCR8 Clinical & Regulatory Monotherapy & Combinations
- Anti-CCR8- Assets by Indication/Phase
- Anti-CCR8 Clinical & Regulatory Timelines
Anti-CCR8 LICENSING, ACQUISITION, AND COLLABORATION DEALS
- Anti-CCR8 Licensing, Acquisition, and Deal values
- Anti-CCR8 Licensing by Transaction type and total amount size by Phases
Anti-CCR8 Pipeline Landscape
- Profile Comparisons At-a-glance
- Anti-CCR8 Pipeline Drug Profiles
- Phase I/II
- BMS-986340 (Bristol-Myers Squibb)
- Product Profile & Description
- Clinical Trials
- Other Developments
- S-531011 (Shionogi)
- Product Profile & Description
- Clinical Trials
- Other Developments
- LM-108 (LaNova Medicines)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- BMS-986340 (Bristol-Myers Squibb)
- Phase I
- ICP B05 (InnoCare Pharma/Keymed Biosciences)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- BAY 3375968 (Bayer)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- GS 1811 (Gilead Sciences)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- IPG 7236 (Nanjing Immunophage Biotech Co., Ltd)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- ABBV 514 (AbbVie)
- Product Profile & Description
- Clinical Trials
- Collaborations
- Other Developments
- ICP B05 (InnoCare Pharma/Keymed Biosciences)
- Pre-clinical
- FPA157 (Amgen)
- Product Profile & Description
- Collaborations
- Other Developments
- HBM1022 (Harbour BioMed)
- Product Profile & Description
- Other Developments
- CCR8 Treg program (Oncurious)
- Product Profile & Description
- Collaborations
- Other Developments
- ZL-1218 (ZAI Lab)
- Product Profile & Description
- Other Developments
- SRF 114 (Surface Oncology)
- Product Profile & Description
- Collaborations
- Other Developments
- BCG 005 (Biocytogen Pharmaceuticals (Beijing) Co., Ltd./LiberoThera Co., Ltd)
- Product Profile & Description
- Collaborations
- Other Developments
- GB2101 (Genor Biopharma Co. Ltd.)
- Product Profile & Description
- Other Developments
- FG-3136 (HiFiBiO Therapeutics/FibroGen)
- Product Profile & Description
- Other Developments
- Collaborations
- CHS-3318 (Coherus BioSciences)
- Product Profile & Description
- Other Developments
- FPA157 (Amgen)
- Discovery
- Anti-CCR8 Antibody (Domain Therapeutics)
- Product Profile & Description
- Other Developments
- Patents Filed in Recent Years
- Anti-CCR8 Antibody (Domain Therapeutics)
- Phase I/II
Anti-CCR8 SWOT Analysis
Anti-CCR8 Products Positioning
Appendix
- About us



