 |
市場調查報告書
商品編碼
1170395
重病流感市場:1次調查(KOL的洞察) - 市場情報 - 流行病學和至2032年的市場預測Severe Influenza| Primary Research (KOL's Insight) | Market Intelligence | Epidemiology & Market Forecast-2032 |
||||||
重病流感的市場2022年的市場佔有率,預計2018年~至2032年,以7.8%的年複合成長率擴大,2032年達到48億美元。
本報告提供全球重病流感市場相關調查,市場現狀,以及病例數趨勢,患者趨勢,競爭產品的市場上地位,市場機會等資訊。
目錄
摘要整理
重病流感疾病的背景
- 疾病定義
- 症狀和原因
- 流感的分類
- 病的診斷
- 生物標記
流行病學的估計和2032年為止的預測
- 主要調查結果
- 重病流感的G7的併發症
- 手法和資料來源
重病流感的流行病學和模式參數的主要的資訊來源
- 德國
- 法國
- 義大利
- 西班牙
- 英國
- 日本
目前治療方法和醫療行為
未滿足需求
新的治療方法
- 開發平台概要
- 重病流感的治療開發平台
- 產品分析
- Baloxavir和Oseltamivir的聯合治療
- SAB 176
- Oplunofusp
- POLB 001
- Zapnometinib
- CC-42344
- 流感A/B抑制劑
- 產品分析
重病流感-費用負擔和處方箋的調查
未來的治療範例
現在及新的治療方法的年度費用
市場預測
- 主要調查結果
- 至2032年的各國市場預測
- 重病流感的G7整體市場(100萬美元),2018年~2032年
- 重病流感的G7整體市場(100萬美元),各治療法,2018年~2032年
各國市場預測
- 美國
各國市場預測
- 德國
- 法國
- 義大利
- 西班牙
- 英國
- 日本
推動市場要素與阻礙因素
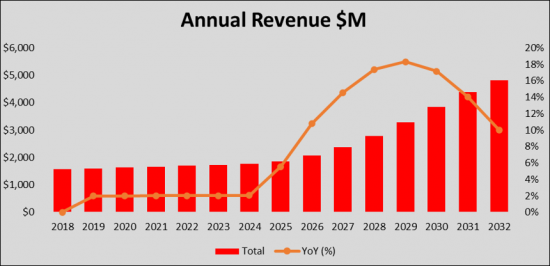
The Severe Influenza market is hugely contributed by the current standard of care (Neuraminidase inhibitor) which is limited to symptom management as there is no approved therapy for Severe Influenza. By 2032, the market is expected to change due to the uptake and launch of new novel therapies. The sales of the emerging therapies for the treatment of Severe Influenza in the study countries (United States, France, Germany, Italy, Spain, United Kingdom, and Japan) will experience high growth over the 2018-2032 study period, adding value estimated at a total market of $ 4.8 billion by 2032.
"Influenza, especially severely ill or severe patients suffer from a major unmet need in terms of efficient treatment. Currently approved vaccines provide 10% to 60% efficacy and approved antivirals often encounter significant viral resistance. Hence, we need an effective treatment to reduce the cases to hospital".
Severe influenza is defined as influenza with a severe symptom or syndrome such as respiratory distress or deceased consciousness or accompanying a severe complication such as encephalopathy or renal failure. Severe influenza presents unique challenges for clinicians. While mild influenza can be treated via ambulatory care, patients who develop complications could get severe influenza which can result in hospitalization and even ICU admission. Infants, the elderly, and sufferers of chronic disease are most at risk of severe influenza, as it can result in complications and exacerbations of underlying conditions. In the UK, children under 5 have the highest influenza admission rate (1.9 per 1000), while over two-thirds (72%) of all influenza-attributable deaths in hospitals occurred in patients over the age of 65 who had co-morbidities.
Severe Influenza - Epidemiology
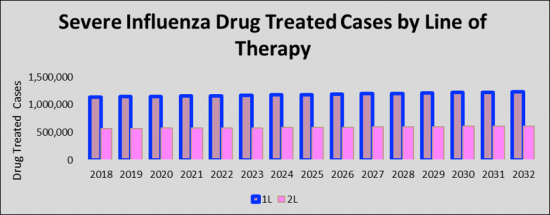
The total Severe Influenza cases in the G7 countries are anticipated to increase to 1,233,662 cases by 2032 for the study period (2018- 2032). As per estimates, the United States accounted for the highest number of Severe Influenza cases in 2018 which was 71,000 cases, and is expected to increase by 2032 for the study period. Among the EU5, Germany had the highest number of Severe Influenza cases, followed by France, the UK, Italy, and Spain. Japan is reported to have the highest number of cases after the United States.
Current treatments for Severe Influenza are limited and, to date, no FDA-approved drugs have proven effective in Severe Influenza. All the patients suffering from severe influenza (SI) will receive the first line of therapy with the initiation of antiviral treatment with oral or enterically-administered oseltamivir, Peramivir, Laninamivir, Baloxavir, and Zanamivir. The drugs have been used alone or in combination to treat Severe Influenza patients with efficiency.

Severe Influenza - Current Market Size & Forecast Trends
The Severe Influenza therapeutics market is expected to experience high growth throughout our study period (i.e. 2018 to 2032) to USD 4.8 billion, representing compound annual growth (CAGR) of 7.8%.
The United States captured the highest market share in 2022 as compared to the European 5 countries and Japan. We expect that with the launch of the new therapies, the current treatment landscape will expand, catering to the need for the treatment of the Severe Influenza patients group. By 2032, the market share of the United States is expected to increase to USD 3.7 billion whereas the European 5 countries and Japan will have USD XX billion and USD XX million market size in 2032, respectively.
In critically ill patients with influenza, early enteral oseltamivir for at least 5 days is the mainstay of antiviral treatment. Treatment duration should be extended if viral tests remain positive. Resistance to oseltamivir should be considered in the case of prolonged persistence. Intravenous peramivir is the alternative when the enteral route cannot be used.
In 2019, EMA approved intravenous (i.v.) zanamivir for the treatment of complicated and potentially life-threatening influenza caused by either influenza A or B viruses in adults and children from 6 months of age. Intravenous zanamivir is an investigational parenterally administered neuraminidase inhibitor product that has been available in the past through enrollment in a clinical trial or under an emergency investigational new drug (EIND) request to the manufacturer. However, since the 2017-18 season, intravenous zanamivir is no longer available in the United States.
In the 2018-2032 forecast period, we expect an increase in the diagnosis rate of Severe Influenza, and usage of the current standard of care including antiviral agents like Neuraminidase inhibitors (NAIs) will drive the Severe Influenza market. The emerging therapies which are expected to enter the market from 2022-2032 period are Combination Therapy Baloxavir + Oseltamivir (Genentech, Inc.), SAB 176 (SAB Biotherapeutics), Oplunofusp (Ansun Biopharma, Inc.), POLB 001 (Poolbeg Pharma).
Report Highlights
- Severe Influenza - Current Market Trends
- Severe Influenza - Current & Forecasted Cases across the G7 Countries
- Severe Influenza - Market Opportunities and Sales Potential for Agents
- Severe Influenza - Patient-based Market Forecast to 2032
- Severe Influenza - Untapped Business Opportunities
- Severe Influenza - Product Positioning Vis-a-vis Competitors' Products
- Severe Influenza - KOLs Insight
Table of Contents
Executive Summary
- Key Findings
Severe Influenza Disease Background
- Disease Definition
- Symptoms & Causes
- Classification of Influenza
- Disease Diagnosis
- Biomarker
Epidemiology Estimated and Forecast to 2032
- Key Findings
- G7 Complications of Severe Influenza
- Methods and Data Sources
- Country Specific cases of Severe Influenza (US, Germany, France, Italy, Spain, UK, and Japan)
- Country Specific First Line (1L) & Second Line 2L Severe Influenza (SI) Treated Cases
- United States
- United States cases of Severe Influenza
- United States First Line (1L) & Second Line 2L Severe Influenza (SI) Treated Cases
Key Sources for Severe Influenza Epidemiology and Model Parameters
- Germany
- Germany cases of Severe Influenza
- Germany First Line (1L) & Second Line 2L Severe Influenza (SI) Treated Cases
- France
- France cases of Severe Influenza
- France First Line (1L) & Second Line 2L Severe Influenza (SI) Treated Cases
- Italy
- Italy cases of Severe Influenza
- Italy First Line (1L) & Second Line 2L Severe Influenza (SI) Treated Cases
- Spain
- Spain cases of Severe Influenza
- Spain First Line (1L) & Second Line 2L Severe Influenza (SI) Treated Cases
- United Kingdom
- United Kingdom cases of Severe Influenza
- United Kingdom First Line (1L) & Second Line 2L Severe Influenza (SI) Treated Cases
- Japan
- Japan cases of Severe Influenza
- Japan First Line (1L) & Second Line 2L Severe Influenza (SI) Treated Cases
Current Therapies and Medical Practice
- Severe Influenza-Recommendations according to WHO Guidelines
- CDC Guideline for considering influenza testing and treatment when influenza viruses are circulating in the community
- CDC Recommendations based on Observational Studies and Experts Opinion
- Recommended Oseltamivir and Peramivir Dose Adjustments for Treatment or Chemoprophylaxis of Influenza in Adult Patients with Renal Impairment or End Stage Renal Disease (ESRD) on Dialysis
- European Treatment Recommendations for the treatment of severe influenza
- Japanese Treatment Guidelines for the treatment of Severe Influenza
Unmet Needs
Emerging Therapies
- Pipeline Overview
- Therapeutic Developments Pipeline for Severe Influenza
- Product Analysis
- Combination Therapy with Baloxavir and Oseltamivir (Genentech, Inc.)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- SAB 176 (SAB Biotherapeutics)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- Oplunofusp (Ansun Biopharma, Inc.)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- POLB 001 (Poolbeg Pharma, Inc.)
- Product Profile
- Clinical Development
- Zapnometinib (Atriva Therapeutics)
- Product Profile
- Clinical Development
- CC-42344 (Cocrystal Pharma Inc)
- Product Profile
- Clinical Development
- Influenza A/B Inhibitor (Cocrystal Pharma Inc/Merck)
- Product Profile
- Combination Therapy with Baloxavir and Oseltamivir (Genentech, Inc.)
- Product Analysis
Severe Influenza- Cost Burden & Prescriptions survey
Future Treatment Paradigm
- Severe Influenza Competitor Landscape and Approvals Anticipated
- Future Treatment Algorithms and Competitor Positioning
Future Treatment Paradigm
- Key Data Summary for Emerging Treatment
- Competitive Landscape of Severe Influenza
- Phase III and Pivotal Phase II Drugs Clinical & Regulatory Timeline in Severe Influenza
- Key Competitor Events 2021-2022
- Annual Cost of Current & Emerging Therapies
Annual Cost of Current & Emerging Therapies
Market Outlook
- Key Findings
- Country Specific Market Forecast to 2032
- G7 total Market for Severe Influenza 2018-2032 (USD Million)
- G7 total Market for Severe Influenza by Therapies 2018-2032 (USD Million)
Market Forecast by Country
- United States
- United States Market for Severe Influenza 2018-2032 (USD Million)
- United States Market for Severe Influenza by Therapies 2018-2032 (USD Million)
Market Forecast by Country
- Germany
- Germany Market for Severe Influenza 2018-2032 (USD Million)
- Germany Market for Severe Influenza by Therapies 2018-2032 (USD Million)
- France
- France Market for Severe Influenza 2018-2032 (USD Million)
- France Market for Severe Influenza by Therapies 2018-2032 (USD Million)
- Italy
- Italy Market for Severe Influenza 2018-2032 (USD Million)
- Italy Market for Severe Influenza by Therapies 2018-2032 (USD Million)
- Spain
- Spain Market for Severe Influenza 2018-2032 (USD Million)
- Spain Market for Severe Influenza by Therapies 2018-2032 (USD Million)
- United Kingdom
- United Kingdom Market for Severe Influenza 2018-2032 (USD Million)
- United Kingdom Market for Severe Influenza by Therapies 2018-2032 (USD Million)
- Japan
- Japan Market for Severe Influenza 2018-2032 (USD Million)
- Japan Market for Severe Influenza by Therapies 2018-2032 (USD Million)
Market Drivers and Constraints
- What Factors Are Driving the Market for Severe Influenza?
- What Factors Are Constraining the Market for Severe Influenza?










