 |
市場調查報告書
商品編碼
1114727
普威二氏症候群(PWS)市場:1次調查(KOL的洞察) - 市場情報 - 流行病學和到2032年為止的市場預測Prader-Willi Syndrome (PWS)| Primary Research (KOL's Insight) | Market Intelligence | Epidemiology & Market Forecast-2032 |
||||||
全球普威二氏症候群(PWS)的市場規模,預計2018年~2032年的調查期間以21.4%的年複合成長率擴大,2032年成為23億5,000萬美元。市場,因為治療方法未確立,目前的標準治療(人體生長荷爾蒙療法)有大幅貢獻。預計2032年,由於新的新治療方法的引進上市,市場將產生變化。
本報告提供全球普威二氏症候群(PWS)市場相關調查,市場現狀,以及病例數趨勢,患者趨勢,競爭產品的市場上地位,市場機會等資訊。
目錄
摘要整理
普威二氏症候群(PWS)疾病的背景
- 普威二氏症候群(PWS)定義
- 症狀和原因
- 病理學
- 診斷
流行病學和至2030年的預測
- 主要調查結果
- 方法和資料來源
- 普威二氏症候群(PWS)的各國盛行率(美國,德國,法國,義大利,西班牙,英國,及日本)
- 普威二氏症候群(PWS)的各國藥物治療病例
- 美國
- 德國
- 法國
- 義大利
- 西班牙
- 英國
- 日本
目前治療方法和醫療行為
未滿足需求
新的治療方法
普威二氏症候群(PWS)- 償付情境
普威二氏症候群(PWS)- 費用負擔與處方箋調查
未來的治療範例
現在及新的治療方法的年度費用
市場預測
各國市場預測
- 美國
- 德國
- 法國
- 義大利
- 西班牙
- 英國
- 日本
市場促進因素與阻礙因素
附錄
The Prader-Willi Syndrome (PWS) market is hugely contributed by current standard of care (human growth hormone therapy) as there is no cure for PWS. By 2032, the market is expected to change due to the uptake and launch of new novel therapies. In a PWS treatment setting, the current SoC will decline, and the novel emerging drugs will grasp the highest market shares. The sales of the emerging therapies for the treatment of PWS in the study countries (United States, France, Germany, Italy, Spain, United Kingdom and Japan) will experience high growth over the 2018-2032 study period, adding a value estimated at a total market of $ 2.1 billion by 2032.
"The most important unmet need for the people with PWS is the treatment of their hyperphagia. This would not only help prevent and reduce morbidity and mortality but could improve the emotional well-being and quality of life of themselves, family members and caregivers. It might also allow the use of less restrictive practices for control of the food environment, saving residential and care costs".
Prader-Willi syndrome (PWS) is a complex developmental genetic disorder associated with hypotonia, poor feeding in neonates, onset of hyperphagia in early childhood, and shorter overall life expectancy. In most people with PWS (about 60%), the PWS/AS region of the father's chromosome 15 is missing or deleted. This chromosomal deletion results from a random error in development and is not inherited (or de novo deletion). Thus, most cases of PWS occur sporadically and the risk of recurrence in another pregnancy is less than 1%. PWS affects males and females in equal numbers and occurs in all ethnic groups and geographic regions in the world. Most estimates place the incidence between 1 in 10,000-30,000 individuals in the general population and about 350,000-400,000 individuals worldwide.
Prader-Willi Syndrome (PWS) - Epidemiology
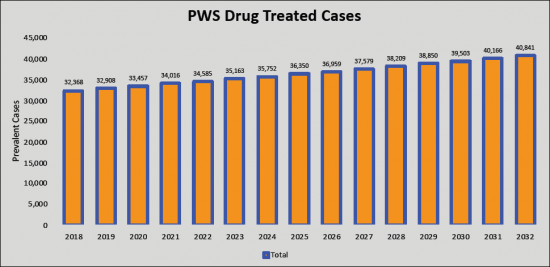
The total PWS prevalent cases in the G7 countries are anticipated to increase to 68,069 cases by 2032 for the study period (2018- 2032). As per estimates, the United States accounted for the highest prevalence of Prader-Willi Syndrome (PWS) cases in 2018 which was 16,463 cases and is expected to increase by 2032 for the study period. Among the EU5, Germany had the highest Prader-Willi Syndrome (PWS) cases, followed by the UK, France, Italy, and Spain. Japan is reported to have the highest number of treated cases after the United States, Germany and UK.
Current treatments for PWS are limited and, to date, focus on the treatment of endocrine abnormalities with hormone replacement therapy. Growth hormone (GH) is FDA-approved for treating children with PWS and is increasingly prescribed in infants and adults. GH is effective in normalizing growth and improving body composition in PWS but has no effect on hyperphagia.

Prader-Willi Syndrome (PWS) - Current Market Size & Forecast Trends
The Prader-Willi Syndrome (PWS) therapeutics market is expected to experience high growth throughout our study period (i.e. 2018 to 2032) to USD 2.35 billion, representing compound annual growth (CAGR) of 21.4%.
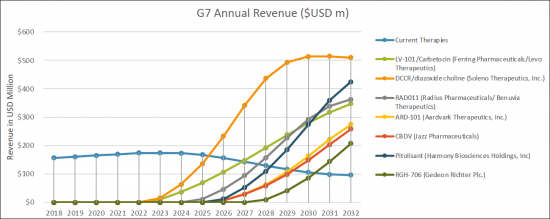
The United States captured the highest market share in 2022 as compared to European 5 countries and Japan. We expect that with the launch of the new therapies, the current treatment landscape will grow, catering to the need of treatment of hyperphagia, anxiousness, distress and excessive day time sleepiness (EDS) associated with Prader-Willi syndrome patients' group. By 2032, the market share of the United States, is expected to increase to USD 1.05 billion whereas European 5 countries and Japan will have USD XX billion and USD XX million market size in 2032, respectively.
In June 2000, HGH was officially approved by the Federal Drug Administration (FDA) in the United States for use in patients with Prader-Willi syndrome. HGH is effective not only in increasing height, but also in decreasing body fat, increasing muscle mass, improving weight distribution, increasing stamina, and increasing bone mineral density. In addition, studies suggest its positive effects on development and behavior.
Despite HGH treatment, many challenging symptoms associated with PWS remain difficult to treat. The inability to control food intake is often the biggest obstacle keeping those with PWS from living independently. To date, no medications have proven effective in regulating appetite in PWS, and therefore, strict environmental control and constant supervision are the only ways to prevent life-threatening overeating and extreme obesity at present.
In the 2018-2032 forecast period, we expect a greater uptake of the new therapies specifically in the treatment of treatment of hyperphagia, anxiousness, distress and excessive day time sleepiness (EDS) associated with Prader-Willi syndrome. The emerging therapies which are expected to enter the market from 2022-2032 period are LV-101/Carbetocin (Ferring Pharmaceuticals/Levo Therapeutics), Diazoxide Choline Controlled-Release/ DCCR (Soleno Therapeutics, Inc.), Synthetic cannabidiol/RAD011 (Radius Pharmaceuticals/ Benuvia Therapeutics), ARD-101 (Aardvark Therapeutics, Inc.), CBDV/cannabidivarin/GWP42006 (Jazz Pharmaceuticals), Pitolisant (Harmony Biosciences Holdings, Inc) and RGH-706 (Gedeon Richter Plc.).

Report Highlights:
- Prader-Willi Syndrome (PWS) - Current Market Trends
- Prader-Willi Syndrome (PWS) - Current & Forecasted Cases across the G7 Countries
- Prader-Willi Syndrome (PWS) - Market Opportunities and Sales Potential for Agents
- Prader-Willi Syndrome (PWS) - Patient-based Market Forecast to 2032
- Prader-Willi Syndrome (PWS) - Untapped Business Opportunities
- Prader-Willi Syndrome (PWS) - Product Positioning Vis-a-vis Competitors' Products
- Prader-Willi Syndrome (PWS) - KOLs Insight
Table of Contents
Executive Summary
- Key Findings
Prader-Willi Syndrome (PWS) Disease Background
- Prader-Willi Syndrome (PWS) Definition
- Symptoms & Causes
- Pathophysiology
- Diagnosis
Epidemiology Estimated and Forecast to 2030
- Key Findings
- Methods and data Sources
- Country Specific Prevalent cases of Prader-Willi Syndrome (PWS) (US, Germany, France, Italy, Spain, UK, and Japan)
- Country Specific Drug Treated Cases of Prader-Willi Syndrome (PWS)
- United States
- United States Prevalent cases of Prader-Willi Syndrome (PWS)
- United States Drug Treated Cases of Prader-Willi Syndrome (PWS)
- Germany
- Germany Prevalent cases of Prader-Willi Syndrome (PWS)
- Germany Drug Treated Cases of Prader-Willi Syndrome (PWS)
- France
- France Prevalent cases of Prader-Willi Syndrome (PWS)
- France Drug Treated Cases of Prader-Willi Syndrome (PWS)
- Italy
- Italy Prevalent cases of Prader-Willi Syndrome (PWS)
- Italy Drug Treated Cases of Prader-Willi Syndrome (PWS)
- Spain
- Spain Prevalent cases of Prader-Willi Syndrome (PWS)
- Spain Drug Treated Cases of Prader-Willi Syndrome (PWS)
- United Kingdom
- United Kingdom Prevalent cases of Prader-Willi Syndrome (PWS)
- United Kingdom Drug Treated Cases of Prader-Willi Syndrome (PWS)
- Japan
- Japan Prevalent cases of Prader-Willi Syndrome (PWS)
- Japan Drug Treated Cases of Prader-Willi Syndrome (PWS)
Current Therapies and Medical Practice
- Treatments & Medical Practices
Unmet Needs
Emerging Therapies
- Pipeline Overview
- Therapeutic Developments Pipeline for Prader-Willi Syndrome (PWS)
- Product Analysis
- Carbetocin (Ferring Pharmaceuticals/Levo Therapeutics)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- DCCR (Soleno Therapeutics, Inc.)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- RAD011 (Radius Pharmaceuticals, Inc.)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- ARD-101 (Aardvark Therapeutics, Inc.)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- CBDV (Jazz Pharmaceuticals)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- Pitolisant (Harmony Biosciences Holdings, Inc)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- RGH-706 (Gedeon Richter Plc.)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- Eutropin (Biopartners/LG Chem)
- Product Profile
- Clinical Development
- Metoprolol/tesofensine (Saniona)
- Product Profile
- Clinical Development
- HBS-102 (Harmony Biosciences Holdings, Inc)
- Product Profile
- Clinical Development
- Carbetocin (Ferring Pharmaceuticals/Levo Therapeutics)
- Product Analysis
Prader-Willi Syndrome (PWS)- Reimbursement Scenario
Prader-Willi Syndrome (PWS)- Cost Burden & Prescriptions survey
Future Treatment Paradigm
- Prader-Willi Syndrome (PWS) Competitor Landscape and Approvals Anticipated
- Future Treatment Algorithms and Competitor Positioning
- Key Data Summary for Emerging Treatment
- Competitive Landscape of Prader-Willi Syndrome (PWS)
- Phase III and Pivotal Phase II Drugs Clinical & Regulatory Timeline in PWS
Annual Cost of Current & Emerging Therapies
Market Outlook
- Key Findings
- Country Specific Market Forecast to 2030
- G7 total Market for Prader-Willi Syndrome (PWS) 2020-2030 (USD Million)
- G7 total Market for Prader-Willi Syndrome (PWS) by Therapies 2020-2030 (USD Million)
Market Forecast by Country
- United States
- United States Market for Prader-Willi Syndrome (PWS) 2020-2030 (USD Million)
- United States Market for Prader-Willi Syndrome (PWS) by Therapies 2020-2030 (USD Million)
- Germany
- Germany Market for Prader-Willi Syndrome (PWS) 2020-2030 (USD Million)
- Germany Market for Prader-Willi Syndrome (PWS) by Therapies 2020-2030 (USD Million)
- France
- France Market for Prader-Willi Syndrome (PWS) 2020-2030 (USD Million)
- France Market for Prader-Willi Syndrome (PWS) by Therapies 2020-2030 (USD Million)
- Italy
- Italy Market for Prader-Willi Syndrome (PWS) 2020-2030 (USD Million)
- Italy Market for Prader-Willi Syndrome (PWS) by Therapies 2020-2030 (USD Million)
- Spain
- Spain Market for Prader-Willi Syndrome (PWS) 2020-2030 (USD Million)
- Spain Market for Prader-Willi Syndrome (PWS) by Therapies 2020-2030 (USD Million)
- United Kingdom
- United Kingdom Market for Prader-Willi Syndrome (PWS) 2020-2030 (USD Million)
- United Kingdom Market for Prader-Willi Syndrome (PWS) by Therapies 2020-2030 (USD Million)
- Japan
- Japan Market for Prader-Willi Syndrome (PWS) 2020-2030 (USD Million)
- Japan Market for Prader-Willi Syndrome (PWS) by Therapies 2020-2030 (USD Million)
Market Drivers and Constraints
- What Factors Are Driving the Market for Prader-Willi Syndrome (PWS)?
- What Factors Are Constraining the Market for Prader-Willi Syndrome (PWS)?




