 |
年間契約型資訊服務
商品編碼
1349830
頭頸鱗狀細胞癌 (HNSCC) - Tumor DeckHead and Neck Squamous Cell Carcinoma (HNSCC) - Tumour Deck |
||||||
頭頸癌 (H&N) 的主要目標是起源於口腔的腫瘤,包括唇黏膜、咽、喉和鼻竇。 這些腫瘤95%以上是鱗狀細胞癌。 口腔癌、下嚥癌、喉癌和非人類乳突病毒 (HPV) 相關口咽癌最常見的原因是吸菸和飲酒障礙。 頭頸癌患者,尤其是菸草和酒精誘發的頭頸癌患者,在頭頸、肺部、食道、膀胱和其他接觸這些致癌物質的部位同步發生原發性腫瘤,存在發生頭頸癌的風險。第二原發腫瘤。
在先前的治療(通常包括放射治療和化療)後疾病進展後,轉移性頭頸癌的治療尤其具有挑戰性。 過去四年,免疫療法的批准和兩種 PD-1 抑制劑用於治療復發轉移的批准令人興奮。 臨床試驗目前正在進行中,並正在走向最終治療。
頭頸鱗狀細胞癌不僅由癌細胞組成,而且是一個動態的生態系統,腫瘤細胞與微環境的各個組成部分相互作用。 這個生態系統包括免疫細胞、癌症相關成纖維細胞 (CAF)、癌症幹細胞 (CSC)、脈管系統和病毒因子,例如人類乳突病毒 (HPV)。 了解這些成分之間的相互作用和串擾對於制定有效的治療策略至關重要。
晚期腫瘤發生率相對較高,可能與HNSCC腫瘤早期患者症狀有限或從早期向晚期進展較快有關。 高達 40% 的 cN0 頸部有隱性轉移性疾病。 因此,開發用於早期檢測轉移的腫瘤生物標記至關重要。 腫瘤標記在二級預防中發揮重要作用。 可以使用生化和免疫學表達作為腫瘤標記來量化腫瘤分化。 目前,經過嚴格的體外測試,FDA 已批准 28 種生物標記用於臨床。 然而,目前還沒有 FDA 批准的 HNSCC 診斷或預後的蛋白質或突變標記物。
接受多種藥物治療的局部晚期 HNSCC 患者中,超過 50% 會在完成治癒性治療後 3 年內復發或轉移。 目前,由於沒有有效的早期發現篩檢方法,大量病例確診時已屬晚期。
本報告調查了全球頭頸鱗狀細胞癌 (HNSCC) 市場,並提供了市場現狀、病例數趨勢、患者趨勢、競爭產品的市場定位以及市場機會。我是。
目錄
第 1 章執行摘要
第 2 章頭頸鱗狀細胞癌 (HNSCC) 概述
- 頭頸鱗狀細胞癌 (HNSCC) 的定義、症狀、病因和發病機制
- HNSCC 的分子變化
第3章頭頸鱗狀細胞癌(HNSCC)的定義與診斷
- 診斷生物標記
- 診斷演算法
第 4 章頭頸鱗狀細胞癌 (HNSCC) 流行病學
- 按國家/地區劃分的盛行率
- HNSCC 國家階段分類
第5章頭頸鱗狀細胞癌(HNSCC)的治療實務
第 6 章核准的頭頸鱗狀細胞癌 (HNSCC) 標靶治療
- 標靶治療概述
- 頭頸鱗狀細胞癌 (HNSCC) 的未滿足需求
- 疾病復發和/或轉移率高
- HPV 陽性和 HPV 陰性 HNSCC 的治療策略沒有差異
- 目前標靶治療面臨的新挑戰
第7章管道臨床試驗
- 頭頸鱗狀細胞癌(HNSCC)管狀情形概述與分析
- 分子標靶治療的臨床進展
- 關鍵分子及臨床試驗結果
- 主要藥物核准與上市的時間表
第8章三期資產
- 臨床試驗和結果
- 二期資產
- 臨床試驗和結果
第9章第一期資產
- 臨床試驗和結果
第 10 章頭頸鱗狀細胞癌 (HNSCC) 研發管線中的早期分子
第 11 章頭頸鱗狀細胞癌 (HNSCC) 研發管線中的非臨床分子
第12章醫生/KOL輸入
- 來自美國、歐盟和日本 4 位 KOL 的見解
- 頭頸鱗狀細胞癌 (HNSCC) 的重大事件
- 擴大已核准的標靶治療
- 訂定新的可行目標
第 13 章頭頸鱗狀細胞癌 (HNSCC) 市場預測 - 2033 年
- 主要藥物的市場預測與病患份額
第14章附錄
Head and Neck (H&N) cancers primarily targets tumors originating from the oral cavity, including the mucosal lip, pharynx, larynx, and paranasal sinuses. More than 95% of these tumors are squamous cell carcinomas. The most common causes for oral cavity, hypopharynx, larynx, and Human Papillomavirus (HPV)-unrelated oropharynx cancers are tobacco and alcohol use disorders. Patients with H&N cancers, particularly those caused by tobacco and alcohol, risk synchronous primary tumors and developing second primary neoplasms in the H&N, lung, esophagus, bladder, and other sites exposed to these carcinogens.
"Metastatic head and neck cancer is a challenging disease to treat particularly after disease progression on prior therapy, which usually includes radiation and chemotherapy. Over the last 4 years, we've seen the big excitement with immunotherapy being approved, with 2 PD-1 inhibitors approved in the recurrent metastatic setting. We're seeing this moving now to the definitive therapy setting with trials that are accruing."
Head and neck squamous cell carcinoma is not solely composed of cancer cells but is rather a dynamic ecosystem where tumor cells interact with various components in their microenvironment. This ecosystem includes immune cells, cancer-associated fibroblasts (CAFs), cancer stem cells (CSCs), vasculature, and viral factors such as human papillomavirus (HPV). Understanding the interactions and crosstalk between these components is essential for developing effective treatment strategies.
The relative higher incidence of advanced stage tumours could be related to limited symptomatology in patients with early stage or swift progression from early to advanced stage in HNSCC tumours. Up to 40% of cN0 necks harbor occult metastatic disease. Hence, developing tumour biomarkers to detect metastasis at early stage is essential. Tumour markers play a significant role in secondary prevention. Tumour differentiation can be quantified using biochemical and immunological representation as tumour markers. Currently, the FDA has approved 28 biomarkers after robust in vitro tests for clinical use. However, there is no protein or mutation marker approved for diagnosis or prognosis in HNSCC by the FDA
Mellalta Meets: Predictive Biomarkers of Checkpoint inhibitors
Predictive Biomarkers of Checkpoint inhibitors
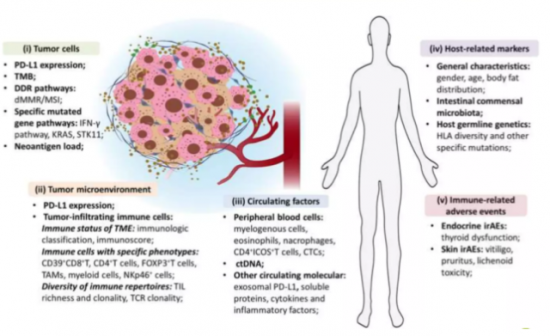
Source: Bai et al, 2020.
Mellalta's Head and neck squamous cell carcinoma (HNSCC) Deck: Current Treatment Landscape
The current standard of care for locally advanced (LA) HNSCC and recurrent/metastatic (R/M) HNSCC involves a combination of treatment modalities, including surgery, radiation therapy (RT), and chemotherapy.
For LA HNSCC, the primary treatment modality is often a combination of surgery and RT. In some cases, multimodal approach is considered in which chemotherapy may also be administered concurrently with RT to enhance its effectiveness.
For R/M HNSCC, the treatment options are more limited. Systemic therapy, which includes chemotherapy and targeted therapy, is the mainstay of treatment. Chemotherapy drugs such as cisplatin, carboplatin, and 5-fluorouracil are commonly used. Targeted therapies, such as cetuximab (an anti-EGFR monoclonal antibody), may also be used in combination with chemotherapy.
Immunotherapy has emerged as a promising treatment option for R/M HNSCC. Immune checkpoint inhibitors, such as pembrolizumab and nivolumab, have shown significant efficacy in improving overall survival in patients with R/M HNSCC.
Mellalta Meets Immunotherapies in HNSCC
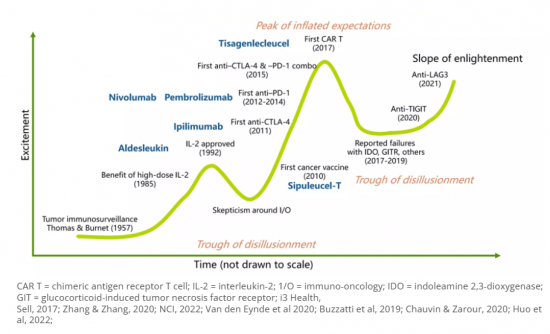
"Management of early-stage locoregional HNSCC primarily rests on a combination of chemotherapy and radiation therapy. However, the therapeutic trajectory becomes intricate for patients experiencing local or regional recurrence due to radiation field overlaps. Additionally, the management of recurrent or second primary HNSCC has become more complex due to the increased incidence of HPV-associated HNSCC compared to non-HPV HNSCC. This change in disease profile has led to a wider range of treatment options available to practicing oncologists, further complicating the decision-making process."
Mellalta's Head and neck squamous cell carcinoma (HNSCC) Deck: Current Unmet Needs
- High rate of disease recurrence and/or metastasis and poor treatment outcomes severely affect long term survival: Over 50% of patients with locally advanced HNSCC who receive multimodal approached develop recurrent or metastatic disease within 3 years of the completing curative-intent treatments.
- Early detection of HNSCC is crucial for better treatment outcomes: Currently, there is a lack of effective screening methods for early detection, leading to a significant number of cases being diagnosed at advanced stages.
- No distinction in treatment strategy for HPV-positive and HPV-negative HNSCC which produces significant morbidity: HPV-positive and HPV-negative HNSCC distinctly differ genetically, they are treated in much the same way, an approach that produces significant morbidity. For patients with advanced-stage HPV-positive HNSCC, the 5-year survival rates are 75% to 80%, whereas fewer than 50% of patients with HPV-negative disease survive for 5 years.
- Diverse cases of Potential ICI resistance mechanisms in R/M HNSCC, which necessitates that many molecular targeted agents be tested in combination with ICIs
"Use of immunotherapy in the treatment of [HNSCC] is still evolving, with a continued unmet need for first-line regimens that provide durable clinical benefit with tolerable safety, further research is needed to determine the utility of dual immunotherapy as a treatment option for [HNSCC] and identify novel biomarkers to predict benefit with immunotherapy."
Mellalta's Head and neck squamous cell carcinoma (HNSCC) Deck: Key Takeaways
- Radiotherapy plus concomitant chemotherapy for fit patient's with unresected LA SCCHN (stage III-IV) has been shown to improve 5-year absolute survival by 6.5% compared with locoregional treatment alone.
- Despite of Cisplatin-Associated Toxicities, Cisplatin remains the radiosensitizer of choice for the majority of patients with locoregionally advanced head and neck cancer.
- Cetuximab is the only targeted therapy that has been proven effective for the treatment of HNSCC in both the LA and R/M settings. The incorporation of cetuximab not only expanded the range of treatment options in the past decade but also encouraged the investigation of many other targeted therapies in this tumor type.
- The current standard of care (SOC) in the second-line treatment of HNSCC typically results in an ORR of approximately 10-18%. There is room for improvement in the effectiveness of existing treatments.
Mellalta's Head and neck squamous cell carcinoma (HNSCC) Deck: Questions Answered:
- How can anti-PD-1/L1 therapies improve outcomes for patients with locally advanced HNSCC? Which combination strategies involving PD1-based therapies can drive change in recurrent/metastatic HNSCC?
- How can Merck Group's IAP antagonist xevinapant change the treatment paradigm for locally advanced HNSCC?
- How do oncologists view the role of Adlai Nortye's anti-PI3K agent buparlisib in the treatment of PD-1-refractory HNSCC?
- What are the key challenges and opportunities in the development of novel therapies for HNSCC, according to KOLs?
- Which companies are best placed to exploit the approach of novel IO-doublet regimens in HNSCC?
Table of Content
1. Executive Summary
- 1.1. Summary of future trends
- 1.2. Potential opportunities to explore.
- 1.3. Drivers/barriers for entry
- 1.4. What's new in HNSCC.
2. Head and neck squamous cell carcinoma (HNSCC) Overview
- 2.1. Head and neck squamous cell carcinoma (HNSCC) definition, symptoms, etiology, Pathogenesis
- 2.2. Molecular Alterations in HNSCC
3. Head and neck squamous cell carcinoma (HNSCC) Definition & Diagnosis
- 3.1. Diagnostic Biomarkers
- 3.2. Diagnostic Algorithm
4. Head and neck squamous cell carcinoma (HNSCC) Epidemiology
- 4.1. Incidence rates by countries
- 4.2. Stage specific cases of HNSCC by countries
5. Head and neck squamous cell carcinoma (HNSCC) Treatment Practices
- 5.1. Current treatment practices
- 5.2. Treatment algorithms
- 5.3. Summarized version of NCCN and ESMO treatment guidelines
- 5.4. Acceptable endpoints for accelerated approval?
6. Head and neck squamous cell carcinoma (HNSCC) Approved Targeted Treatments
- 6.1. Quick overview of targeted therapies
- 6.2. Unmet Needs in Head and neck squamous cell carcinoma (HNSCC)
- 6.3. High rate of disease recurrence and/or metastasis
- 6.4. No distinction in treatment strategy for HPV-positive and HPV-negative HNSCC
- 6.5. Challenges with the Current Emerging Targeted Therapy
- 6.6. To be Continued…
7. Pipeline clinical trials
- 7.1. Head and neck squamous cell carcinoma (HNSCC) pipeline landscape overview and analysis
- 7.2. Clinical Development of Molecular Targeted Therapy
- 7.3. Key molecules in clinical trials and results
- 7.4. Timeline of key drug approvals and launches.
8. Phase III Assets
- 8.1. Clinical trials and results
- 8.2. Phase II Assets
- 8.3. Clinical trials and results
9. Phase I Assets
- 9.1. Clinical trials and results
10. Head and neck squamous cell carcinoma (HNSCC) Pipeline Early-Stage Molecules
- 10.1. Phase I and Phase I/II molecules
- 10.2. Mechanism of action, trial dates, and topline results
11. Head and neck squamous cell carcinoma (HNSCC) Pipeline Non-clinical Molecules
- 11.1. Pre-clinical molecules
- 11.2. Mechanism of action, catalyst dates, and events
12. Physicians/KOLs Input
- 12.1. Insights from 4 KOLs in the US, EU, and Japan
- 12.2. Key Catalyst Events in Head and neck squamous cell carcinoma (HNSCC)
- 12.3. Expansion of approved targeted therapies
- 12.4. Creation of new actionable targets
13. Head and neck squamous cell carcinoma (HNSCC) Market Forecast -2033
- 13.1. Market Forecast and patient share by key drugs







