 |
市場調查報告書
商品編碼
1069326
CAR-T 細胞治療市場:知識產權趨勢CAR-T Cell Therapies: Intellectual Property Landscape (Featuring Historical and Contemporary Patent Filing Trends, Prior Art Search Expressions, Patent Valuation Analysis, Patentability, Freedom to Operate, Pockets of Innovation, |
||||||
實例洞察
知識產權概述
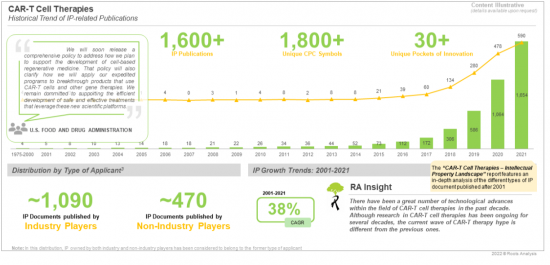
自2000年以來,隨著細胞治療相關法規的相對明晰,1600多項專利/專利涵蓋各類CART-T細胞療法。申請已完成/申請。
目錄
Excel 可交付成果
第一章調查筆記
第 2 章摘要儀表板
第三章整體知識產權情況數據集
第4章現有技術搜索表達式(關鍵字分析)
第五章專利申請數據集
第6章授權專利數據集
第7章計費分析
第八章重點申請人分析
第9章CPC分析
第10章附錄一:數據透視表
第11章附錄二:國家/地區代碼
第十二章附錄三:創新類
PowerPoint 可交付成果
第一章背景
第2章項目方法
第三章項目目的
第 4 章執行摘要
第5章CAR-T細胞療法
- 概覽
- 發展歷程
- FDA 批准的 CAR-T 細胞療法
- 主要優點和局限性
- 未來展望
第六章知識產權總體情況
第 7 章主要現有技術檢索
第八章知識產權評估分析
- 評估概述
- 個人價值排名分析
- 排名第一的IP文檔
- 2 級 IP 文檔
- 3級IP文檔
- 4級IP文檔
- 5級IP文檔
- 結束語
第九章專利申請分析
第十章授權專利分析
第11章主要申請人
第 12 章創新口袋和空白
- 概覽
- 創新口袋
- 空格
- 結束語
第13章未來展望
第14章附錄
公司名單
Title:
CAR-T Cell Therapies:
Intellectual Property Landscape (Featuring Historical and Contemporary Patent Filing Trends, Prior Art Search Expressions, Patent Valuation Analysis, Patentability, Freedom to Operate, Pockets of Innovation, Existing White Spaces, and Claims Analysis).
Example Insights:
Overall Intellectual Property Landscape
Since 2000, over 1,600 patents/patent applications, covering diverse types of CART-T cell therapies, have been granted/filed as regulations related to cell therapies have evolved and become relatively clearer.

Overview
Cancer, one of the leading causes of death worldwide, claimed close to 10 million lives annually Although there are several treatment options available to control disease progression and keep malignant cells from spreading throughout the body, lasting remission is difficult to achieve. In this context, immunotherapies, a relatively recent addition to the gamut of anticancer interventions, have demonstrated significant promise. For instance, after multiple rounds of chemotherapy, a stem cell transplant, participating in two clinical trials of experimental anticancer interventions and targeted radiation therapy, Scott McIntyre was treated with a CAR T-cell therapy at the University of Chicago Medicine, in 2016. Till date, he is in complete remission from his diffuse large B-cell lymphoma (DLBCL). This highly specific and promising form of treatment that harnesses the versatile effector machinery of the human immune system, has revolutionized cancer treatment across the world. CAR-T therapies have so far been evaluated and approved for several hematological malignancies; ABECMA® (relapsed or refractory multiple myeloma) , BREYANZI® (relapsed or refractory large B-cell lymphoma) , TECARTUS™ (relapsed or refractory B-cell precursor ALL) , KYMRIAH™ (relapsed or refractory DLBCL and relapsed or refractory ALL), YESCARTA™ (DLBCL, primary mediastinal B-cell lymphoma, high grade B-cell lymphoma, and follicular lymphoma) , are products involving the use of CAR-T cells, which have been approved by the US FDA.
Presently, the success rate for CAR T-cell therapies is estimated to be around 30% to 40%, offering lasting remission without requiring any additional treatments. Cytokine release syndrome (CRS), a commonly reported side effect of the treatment, is typically observed within a few days to a couple of weeks after CAR T-cell infusion. In severe cases, patients with CRS may need to be transferred to intensive care and even require life-support machines to stay alive. However, over time, medical science has developed the necessary means to control / treat CRS. In addition, a successful CAR-T cell graft is known to cause certain rare types of infections, which are generally observed in severely immunodeficient patients. This is because the programmed, effector T-cells also eliminate normal immune cells, which are responsible for keeping infections at bay, in a healthy host. Other barriers to therapeutic success include inadequate anti-tumor activity (in certain cases), antigen escape, restricted trafficking, and limited tumor infiltration. As a result, there is a lot happening in terms of innovation related to this promising segment of cell-based therapies; medical researchers are developing targeted interventions for different indications and also exploring ways to make the therapy safer. It is also worth mentioning that a lot of capital has also been invested to support R&D activity in this burgeoning field of research. This report attempts to identify key trends that describe the pace and focus of innovation related to CAR-T cell therapies, and make key observations / inferences regarding the development of intellectual capital in this domain.
Scope of the Report
The "CAR-T Cell Therapies: Intellectual Property Landscape" report features an extensive study of the historical and current collection of granted patents, patent applications and affiliated documents associated with the upcoming suite of programmable, personalized anticancer therapies. The information in this report has been presented across two deliverables, namely a MS Excel sheet, featuring an interactive dashboard, and an MS PowerPoint presentation, summarizing the ongoing activity in this domain, and key insights drawn from the available data. The report features the following details:
Overall Intellectual Property Landscape
An in-depth review of the various patents and affiliated IP documents that have been published related to technologies and methods associated with the therapeutic applications of CAR-T cells, featuring key insights on historical and recent trends.
Popular / Relevant Prior Art Search Expressions
An examination of IP literature, including a shortlist of key words and phrases that have been used to describe innovations involving CAR-T cells that are indicated for the treatment of different types of cancers. The analysis also features historical usage trends of the aforementioned terms in IP filings, key affiliated terms (which can be used to further identify similar innovations), and other related details.
Patent Valuation Analysis
A competitive benchmarking and valuation analysis of the IP documents published in this field of innovation, taking into account important parameters, such as type of IP document, year of application, time to expiry, number of citations and jurisdiction (factoring in regional GDP).
Patentability and Freedom to Operate
A systematic approach to identify relevant areas of innovation by analyzing published IP documents, defining the uniqueness of patented / patent pending innovations, understanding the scope of patentability in this domain, and pinpointing jurisdictions where new and / or modified claims may be filed without infringing on existing IP.
Analysis of Patent Applications
A detailed summary of the patent applications that were filed across different jurisdictions and their relative value in the IP ecosystem. The analysis segregates the intellectual capital in terms of area of innovation and intended applications, thereby, offering the means to understand key areas of research and identify innovation-specific IP filing trends.
Analysis of Granted Patents
An elaborate summary of the granted patents across different jurisdictions and their relative value in the IP ecosystem. The analysis segregates the intellectual capital in terms of area of innovation and intended applications; this offers the means to identify unique innovations that presently have marketing exclusivity, and the feasibility for innovators to enter into promising product markets.
Pockets of Innovation and White Spaces
An insightful analysis of the various CPC symbols mentioned in published IP literature and their affiliated families, in order to identify historical and existing pockets of innovation (based on the functional area / industry described by the elaborate and systematic system of classifying IP) ; the analysis also features a discussion on the prevalent white spaces (based on CPC symbols) in this area of research.
Claims Analysis
One of the objectives of the report was to analyze and summarize key inferences from the independent claims mentioned in granted, active patents in the dataset. Using a systematic segregation approach, we have analyzed trends associated with [A] the preamble, [B] type of patent (technology patent or method patent), [C] type of claim (open ended claim or closed ended claim) and [D] key elements of a claim (individual aspects of an innovation that are covered in a singular claim).
DELIVERABLE OUTLINES:
Excel Deliverable
Sheet 1 features details regarding how the input data for this project was collated, including the search strings used to query a popular patent database (lens.org) , and data segregation notes.
Sheet 2 is a summary MS Excel dashboard, offering a detailed graphical perspective of the intellectual property landscape of CAR-T cell therapies. It includes pictorial representations of the [A] overall patent landscape, [B] key prior art search expressions, [C] trends related to patent applications (including insights on patentability and freedom to operate) , [D] trends related to granted patents (including insights on patentability and freedom to operate) , [E] key inferences from a proprietary claims analysis, [F] list of popular CPC symbols (featuring key pockets of innovation) , [G] list of popular applicants (shortlisted based on number of published IP documents) .
Sheet 3 is an elaborate tabular representation of the overall IP landscape, featuring information on the various patent application- and granted patent-related documents that have been published since 2000.
Sheet 4 includes a tabular representation of key words and phrases that are used to describe CAR-T cell therapies.
Sheet 5 is a subset of sheet 3, featuring all the patent applications, covering intellectual capital related to CAR-T cell therapies.
Sheet 6 is a subset of sheet 3, featuring all the granted patents, covering intellectual capital related to CAR-T cell therapies.
Sheet 7 is an insightful summary of key inferences from the independent claims mentioned in the granted, active patents in the dataset. We have used a systematic segregation approach to analyze trends associated with the preamble, type of patent (technology patent or method patent), type of claim (open ended claim or closed ended claim) and key elements of a claim (individual aspects of an innovation that are covered in a singular claim).
Sheet 8 provides insights related to some of the key applicants in this field of research, featuring company-specific details (such as year of establishment, and location of headquarters), and inputs on their respective IP publication trends.
Sheet 9 features an analysis of the most popular (in terms of frequency of appearance in the dataset) CPC symbols and CPC families, related to CAR-T cell therapies.
Sheet 10 is an appendix which includes pivot tables that drive the charts and interactive elements for the complete IP landscape, in sheet 2.
Sheet 11 is an appendix, featuring details related to the categorization done in the report, and important abbreviations used in reference to the data categories mentioned in the document.
PowerPoint Deliverable
Chapter 1 briefly describes the need for better therapeutic options in the field of cancer treatment, and the key advantages of CAR-T cell therapies. It also features an overview of the intellectual property landscape related to this upcoming class of advanced therapy medicinal products.
Chapter 2 and 3 feature brief (pictorial) summaries of the approach used for data collection and the key objectives of the study.
Chapter 4 features an executive summary of the key insights generated from analyzing the intellectual property landscape of CAR-T cell therapies.
Chapter 5 provides a brief overview of T-cell therapies, the concept of chimeric antigen receptors and their applicability in personalized medicine. It also features a summary of the key events and milestones associated with the development of one of the most versatile classes of immunotherapies. The chapter lists the various US FDA approved CAR-T cell therapies and their key benefits and limitations. It concludes with a brief description of the likely future of this class of biological interventions. It is worth mentioning that the inputs featured in this chapter were validated based on the opinions of key experts involved in this segment of the biopharmaceutical industry.
Chapter 6 includes a review of the various patents and IP documents that have been published describing various technologies and methods associated with CAR-T cell therapies, featuring key insights on historical and recent trends.
Chapter 7 features an insightful examination of IP literature, identifying key words and phrases that have been / are being used to describe CAR-T cell therapies. The chapter includes information on historical usage of the aforementioned expressions in IP filings, key affiliated terms (which can be used to further identify similar innovations), and other related trends.
Chapter 8 offers insights generated from a competitive benchmarking and valuation analysis of the IP documents related to CAR-T cell therapies, taking into account important parameters, such as type of IP document, year of application, time to expiry, number of citations and jurisdiction (factoring in regional GDP).
Chapter 9 provides a detailed summary of the patent applications that were filed across different jurisdictions and their relative value in the IP ecosystem. The analysis segregates the intellectual capital in terms of area of innovation and intended applications, thereby, offering the means to understand key areas of research and identify innovation-specific IP filing trends. Based on a systematic approach, the chapter highlights relevant areas of innovation from a deeper analysis of published IP documents, defining the uniqueness of patent pending innovations, understanding the scope of patentability in this domain, and pinpointing jurisdictions where new and / or modified claims may be filed without infringing on existing IP.
Chapter 10 is an elaborate summary of the granted patents across different jurisdictions and their relative value in the IP ecosystem. The analysis uses a segregation criterion, based on type of product / solution and intended applications; this offers the means to identify unique innovations that presently have marketing exclusivity and explore future opportunities to enter into promising product markets, once their patents expire. Based on a systematic approach, the chapter highlights relevant areas of innovation from a deeper analysis of published IP documents, defining the uniqueness of patented innovations, understanding the scope of patentability in this domain, and pinpointing jurisdictions where new and / or modified claims may be filed without infringing on existing IP.
Chapter 11 features profiles of some of the most popular applicant companies, which were shortlisted based on patent filing activity. Each profiles includes, a brief overview of the company, information on annual revenues (wherever available), details of its initiatives focused on the healthcare sector, names of key management team members and recent developments.
Chapter 12 includes an insightful analysis of the various CPC symbols mentioned in the published IP literature and their affiliated families, in order to identify historical and existing pockets of innovation (based on the functional area / industry described by the elaborate and systematic system of classifying IP); the analysis also features a discussion on the prevalent white spaces (based on CPC symbols) in this area of research.
Chapter 13 concludes the report by providing insights on anticipated developments in this domain (from the perspective of eminent industry representatives of stakeholders in this domain) , and trends that are likely to shape the future of the CAR-T cell therapies market.
Chapter 14 is a set of appendices.
Table of Contents
Excel Deliverable
1. Research Notes
2. Summary Dashboard
- A. Overall Intellectual Property Landscape
- B Key Prior Art Search Expressions
- C. Key Trends related to Patent Applications (featuring Patentability & Freedom to Operate)
- D. Key Trends related to Granted Patents (featuring Patentability & Freedom to Operate)
- E. Claims Analysis
- F. Key CPC Symbols
- G. Key Applicants
3. Overall Intellectual Property Landscape Dataset
4. Prior Art Search Expressions (Keyword Analysis)
5. Patent Applications Dataset
6. Granted Patents Dataset
7. Claims Analysis
8. Key Applicants Analysis
9. CPC Analysis
10. Appendix I: Pivot Tables
11. Appendix II: Country / Geography Codes
12. Appendix III: Innovation Categories
PowerPoint Deliverable
1. Context
2. Project Approach
3. Project Objectives
4. Executive Summary
5. CAR-T cell therapies
- 5.1. Overview
- 5.2. History of Development
- 5.3. FDA Approved CAR-T cell therapies
- 5.4. Key Benefits and Limitations
- 5.5. Future Perspectives
6. Overall Intellectual Property Landscape
- 6.1. Overview
- 6.2. Analysis of Published IP Documents
- 6.3. Insights from Patent Applications
- 6.4. Insights from Granted Patents
7. Key Prior Art Search Expressions
- 7.1. Overview
- 7.2. Analysis of Prior Art Search Expressions
8. Intellectual Property Valuation Analysis
- 8.1. Valuation Overview
- 8.2. Analysis of Individual Value Ranks
- 8.2.1. Rank 1 IP Documents
- 8.2.2. Rank 2 IP Documents
- 8.2.3. Rank 3 IP Documents
- 8.2.4. Rank 4 IP Documents
- 8.2.5. Rank 5 IP Documents
- 8.3. Concluding Remarks
9. Analysis of Patent Applications
- 9.1. Overview
- 9.2. Relative Valuation of Patent Applications
- 9.3. Patentability & Freedom-to-Operate
10. Analysis of Granted Patents
- 10.1. Overview
- 10.2. Relative Valuation of Grated Patents
- 10.3. Patentability & Freedom-to-Operate
- 10.4. Analysis of Patent Claims
11. Key Applicants
- 11.1. Overview
- 11.2. Analysis of Key Applicants
- 11.2.1. University of Pennsylvania
- 11.2.2. Juno Therapeutics
- 11.2.3. Cellectis
- 11.2.4. University of California
- 11.2.5. bluebird bio
- 11.2.6. Memorial Sloan Kettering Cancer Center
- 11.2.7. Novartis
- 11.2.8. Baylor College of Medicine
- 11.2.9. Kite Pharma
- 11.2.10. University of Texas
12. Pockets of Innovation and White Spaces
- 12.1. Overview
- 12.2. Pockets of Innovation
- 12.3. White Spaces
- 12.4. Concluding Remarks
13. Future Outlook
- 14.1. Overview
- 14.2. Contemporary Sentiments & Expert Opinions
- 14.3. Anticipated Future Developments & Trends
14. Appendices
List Of Companies
The following companies / institutes / government bodies and organizations have been mentioned in this report.
- 1. A2 Biotherapeutics
- 2. AbClon
- 3. Abion
- 4. Accurus Biosciences
- 5. Acerta Pharma
- 6. Achelois Oncology
- 7. Adaptimmune
- 8. Adicet Bio
- 9. Affyimmune Therapeutics
- 10. Agency for Science, Technology and Research (A*STAR)
- 11. Akeso Therapeutics
- 12. Albert Einstein College of Medicine
- 13. Aleta BioTherapeutics
- 14. Allogene Therapeutics
- 15. Almac
- 16. Alpine Immune Sciences
- 17. Altor Bioscience
- 18. Ambrx
- 19. American Gene Technologies
- 20. Amgen
- 21. Ampsource Biopharma Shanghai
- 22. Apceth Biopharma
- 23. Arcellx
- 24. ARMO Biosciences
- 25. Asclepius Technology Company Group (Suzhou)
- 26. Asimov
- 27. Atara Biotherapeutics
- 28. Atossa Genetics
- 29. Autolus Therapeutics
- 30. Auxolytic
- 31. Avacta Life Sciences
- 32. Avectas
- 33. Ayala Pharmaceuticals
- 34. BCell Solutions
- 35. Batu Biologics
- 36. Baylor College of Medicine
- 37. Beam Therapeutics
- 38. Beijing Marino Biotechnology
- 39. Bellicum Pharmaceuticals
- 40. Ben-Gurion University of the Negev
- 41. Benitec Biopharma
- 42. Berkeley Lights
- 43. BioMed Valley Discoveries
- 44. BioNTech
- 45. Biotheus
- 46. bluebird bio
- 47. Board of Regents - University of Texas System
- 48. Boston 3T Biotechnologies
- 49. Brigham and Women's Hospital
- 50. Broad Institute
- 51. Cafa Therapeutics
- 52. California Institute for Biomedical Research
- 53. California Institute of Technology
- 54. Cancer Research Technology
- 55. Cancer Targeting Systems
- 56. Carina Biotech
- 57. CARsgen Therapeutics
- 58. Cartesian Therapeutics
- 59. CARTEXELL
- 60. Cartherics
- 61. Case Western Reserve University
- 62. Celgene
- 63. Cell Design Labs
- 64. Cell Medica
- 65. Celldex Therapeutics
- 66. Cellect
- 67. Cellgentek
- 68. Celllular Biomedicine Group
- 69. Cellular Therapeutics
- 70. Cellyan Therapeutics
- 71. Celularity
- 72. CERo Therapeutics
- 73. Cerus Corporation
- 74. Children's Healthcare of Atlanta
- 75. Connecticut Children's Medical Center
- 76. Chimera Bioengineering
- 77. Chongqing Precision Biotech
- 78. COARE Biotechnology
- 79. Constant Therapeutics
- 80. Council of the Queensland Institute of Medical Research
- 81. Crescendo Biologics
- 82. Crispr Therapeutics
- 83. CTG Pharma
- 84. Cue Biopharma
- 85. CureGenetics
- 86. Curocell
- 87. Cynata Therapeutics
- 88. CytoImmune Therapeutics
- 89. Dana-Farber Cancer Institute
- 90. DaRen
- 91. Dartmouth College
- 92. Diagnologix
- 93. DINONA
- 94. Dorian Therapeutics
- 95. Dragonfly Therapeutics
- 96. DRK-Blutspendedienst Baden-Württemberg - Hessen gGmbH
- 97. East Tennessee State University
- 98. EdiGene
- 99. Editas Medicine
- 100. ElevateBio
- 101. Elstar Therapeutics
- 102. Endocyte
- 103. Enlivex Therapeutics
- 104. Enochian Biopharma
- 105. EpiVax
- 106. Etubics Corporation
- 107. EUCHLOE BIO
- 108. Eureka Therapeutics
- 109. Eutilex
- 110. Exuma Biotechnology
- 111. F1 Oncology
- 112. Fate Therapeutics
- 113. Five Prime Therapeutics
- 114. Flagship Pioneering
- 115. FloDesign Sonics
- 116. San Raffaele Hospital
- 117. Tettamanti Foundation
- 118. Telethon Foundation
- 119. Forevertek Biotechnology
- 120. Fortis Therapeutics
- 121. Forty Seven
- 122. Fujifilm Cellular Dynamics
- 123. Josep Carreras Leukemia Research Institute
- 124. Fundamenta Therapeutics
- 125. Wuling(Fuzhou) Biotechnology
- 126. GammaDelta Therapeutics
- 127. GAVISH - GALILEE BIO APPLICATIONS
- 128. GE Healthcare Systems
- 129. GEMoaB Monoclonals
- 130. Genentech
- 131. Geneuin-Tech
- 132. GigaMune
- 133. GlaxoSmithKline
- 134. Good T cells
- 135. GPB Scientific
- 136. Gracell Biotechnologies
- 137. Green Cross LabCell
- 138. Gritstone Oncology
- 139. TCRCure Biopharma
- 140. Guangzhou Bio-gene Technology
- 141. Guangzhou Institute of Biomedicine and Health
- 142. Moffitt Cancer Center
- 143. HaemaLogiX
- 144. Hangzhou ConVerd
- 145. U-Cell Therapeutics
- 146. Harpoon Therapeutics
- 147. Harvard College
- 148. Health Research
- 149. Hebei Senlang Biotechnology
- 150. Heinrich Heine University Düsseldorf
- 151. Helix BioPharma
- 152. Helmholtz Zentrum München
- 153. Henry M. Jackson Foundation for the Advancement of Military Medicine
- 154. HRYZ Biotech
- 155. Hunan HuiRui Pharmaceutical
- 156. Humanigen
- 157. Igm Biosciences
- 158. Imel Biotherapeutics
- 159. Immatics Biotechnologies
- 160. Immatics Us
- 161. IMMPACT
- 162. Immune Design
- 163. Immungene
- 164. Immunocore
- 165. Immunomedics
- 166. Immunotech Biopharm
- 167. Imperial College of Science, Technology and Medicine
- 168. IN8bio
- 169. Indian Institute of Technology Bombay
- 170. Inhibrx
- 171. Innovative Cellular Therapeutics
- 172. Inscripta
- 173. Instil Bio
- 174. Cancer Research Institute
- 175. Intellia Therapeutics
- 176. Intima Bioscience
- 177. Intrexon
- 178. Io Therapeutics
- 179. Iovance Biotherapeutics
- 180. Janssen Biotech
- 181. Javelin Oncology
- 182. Jazz Pharmaceuticals
- 183. Johannes Gutenberg University Mainz
- 184. JOHNPRO BIOTECH
- 185. JSR Corporation
- 186. Cellectis
- 187. Juno Therapeutics
- 188. JUNTEN BIO
- 189. Jura Bio
- 190. Kangstem Biotech
- 191. King's College London
- 192. Kite Pharma
- 193. Hauner'sches Children's Hospital
- 194. Korea Advanced Institute of Science and Technology
- 195. Korea Reseach Institute of Bioscience & BioTechnology
- 196. KSQ Therapeutics
- 197. Kumquat Biosciences
- 198. Kyoto Prefectural University
- 199. La Jolla Institute for Immunology
- 200. Leucid Bio
- 201. Life Technologies
- 202. LionTCR
- 203. Lonza Cologne
- 204. Lonza
- 205. Lorantis
- 206. Ludwig Institute for Cancer Research
- 207. Ludwig Maximilian University of Munich
- 208. Lycera
- 209. Mabimmune
- 210. MabSpace Biosciences
- 211. MIT - Massachusetts Institute of Technology
- 212. Maxcyte
- 213. Mayo Foundation For Medical Education
- 214. Med Manor Organics
- 215. MEDICAL & BIOLOGICAL LABORATORIES
- 216. Medical College of Wisconsin
- 217. Medicenna Therapeutics
- 218. Medigene
- 219. Medimmune
- 220. Medinet
- 221. Medisix Therapeutics
- 222. MicroCures
- 223. Millennium Pharmaceuticals
- 224. Miltenyi Biotec
- 225. Minerva Biotechnologies
- 226. Mingsight Pharmaceuticals
- 227. Modernatx
- 228. MOGAM Institute for Biomedical Research
- 229. Monarch Biosciences
- 230. MUSC Foundation for Research Development
- 231. Mustang Bio
- 232. MVZ Prof. Dr. med. Niendorf Pathologie Hamburg-West
- 233. BioHeng
- 234. Nanjing Kaedi Biotech
- 235. Nanjing Legend Biotech
- 236. NanoTomer
- 237. Nantbio
- 238. Nantcell
- 239. Nantkwest
- 240. The National Institute for Biotechnology in the Negev
- 241. Chungbuk National University
- 242. Nagoya University
- 243. NUS - National University of Singapore
- 244. Shiga University of Medical Science
- 245. NDSU Research Foundation
- 246. Nectintx
- 247. NeoImmuneTech
- 248. Neon Therapeutics
- 249. NexImmune
- 250. Nkarta
- 251. Noile-Immune Biotech
- 252. Novartis
- 253. Novoscope Ip
- 254. Oaiscell Biotechnologies
- 255. Obsidian Therapeutics
- 256. Innovation Ohio
- 257. OncoHost
- 258. Oncoimmune
- 259. Oncorus
- 260. OncoTab
- 261. OncoTherapy Science
- 262. ONK Therapeutics
- 263. Oncimmune
- 264. Orig3n
- 265. OxfordBiomedica
- 266. Pact Pharma
- 267. Pharos Vaccine
- 268. Pieris Pharmaceuticals
- 269. Poseida Therapeutics
- 270. Precision BioSciences
- 271. Precision NanoSystems
- 272. Precigen
- 273. Promab Biotechnologies
- 274. Protelica
- 275. Provincial Health Services Authority
- 276. PsiOxus Therapeutics
- 277. Purdue Research Foundation
- 278. QT Holding
- 279. Qu Biologics
- 280. Quell Therapeutics
- 281. Rambam MedTech
- 282. Refuge Biotech
- 283. Regen BioPharma
- 284. Regeneron Pharmaceuticals
- 285. Research Institute at Nationwide Children's Hospital
- 286. Rinat Neuroscience
- 287. Shanghai JiaoTong University Ruijing Hospital
- 288. Russell Biotech
- 289. Sangamo Therapeutics
- 290. AZABU UNIVERSITY
- 291. Scripps Research
- 292. Senti Biosciences
- 293. Shanghai Cell Therapy Group
- 294. Genbase-Shanghai Genbase Biotechnology
- 295. unicar-therapy
- 296. Shattuck Labs
- 297. Shenzhen Pregene Biopharma
- 298. Memorial Sloan Kettering Cancer Center
- 299. Sorrento Therapeutics
- 300. SpringWorks Therapeutics
- 301. SQZ Biotech
- 302. St. Jude Children's Research Hospital
- 303. Netherlands Cancer Institute
- 304. Sunshine Lake Pharma
- 305. Syndax Pharmaceuticals
- 306. SYNIMMUNE
- 307. Syno Minicircle Biotechnology
- 308. Takara Bio
- 309. Takeda
- 310. Tayu Huaxia Biotech Medical Group
- 311. TC BioPharm
- 312. TCR2 Therapeutics
- 313. TCRCure
- 314. Technion Research & Development Foundation
- 315. Technical University of Munich
- 316. tella
- 317. Temple University-of The Commonwealth System of Higher Education
- 318. Terumo Global
- 319. Tessa Therapeutics
- 320. The Texas A&M University System
- 321. Fourth Military Medical University
- 322. Massachusetts General Hospital
- 323. Nemours Foundation
- 324. Trustees of the University of Pennsylvania
- 325. University of Notre Dame
- 326. TheryCell
- 327. Thyas
- 328. Timmune Biotech
- 329. Torque Therapeutics
- 330. Tri-Institutional Therapeutics Discovery Institute
- 331. Translational Oncology at the University Medical Center of Johannes Gutenberg University Mainz
- 332. University of Alabama at Birmingham
- 333. UCL Business
- 334. Umoja Biopharma
- 335. Aix-Marseille University
- 336. University of Alberta
- 337. University of Arizona
- 338. Bar-Ilan University
- 339. University of Basel
- 340. Charite University Hospital
- 341. University of Birmingham
- 342. University of Bonn
- 343. Boston University
- 344. Braunschweig University of Technology
- 345. University of British Columbia
- 346. Vrije Universiteit Brussel
- 347. University of California
- 348. University of Chicago
- 349. University of Colorado Board of Regents
- 350. Columbia University
- 351. Cornell University
- 352. Dankook University
- 353. University of Évry Val d'Essonne
- 354. Duke University
- 355. Emory University
- 356. University of Florida
- 357. University of Freiburg
- 358. Fudan University
- 359. University of Geneva
- 360. Ghent University
- 361. The George Washington University
- 362. University Health Network (UHN)
- 363. University of Houston System
- 364. University of Illinois Urbana-Champaign
- 365. Indiana University Research & Technology
- 366. University of Iowa Research Foundation
- 367. Thomas Jefferson University
- 368. Johns Hopkins University
- 369. University of Kansas
- 370. Keio University
- 371. University of Cologne
- 372. Kyoto University
- 373. Stanford University
- 374. Loyola University
- 375. Autonomous University of Madrid
- 376. University of Maryland, College Park
- 377. University of Massachusetts
- 378. China Medical University
- 379. University of Miami
- 380. Regents of the University of Michigan
- 381. Mie University
- 382. University of Minnesota
- 383. Monash University
- 384. Université de Montréal
- 385. University of Nantes
- 386. National University Corporation Kochi University
- 387. University of Nebraska-Lincoln
- 388. The University of North Carolina at Chapel Hill
- 389. University of North Carolina at Charlotte
- 390. Northwestern University
- 391. University of Nottingham
- 392. University of Lausanne
- 393. University of Southern Denmark
- 394. University of Oslo
- 395. Oxford University Innovation
- 396. University of Paris
- 397. University of Pennsylvania
- 398. University of Pittsburgh
- 399. Princeton University
- 400. University of Regensburg
- 401. Rice University
- 402. University of Rouen
- 403. Rutgers University
- 404. Sapporo Medical University
- 405. Seoul National University
- 406. Shinshu University
- 407. SiChuan University
- 408. University of Southampton
- 409. University of Southern California
- 410. University of Strasbourg
- 411. University of Tennessee
- 412. University of Texas at Austin
- 413. University of Toledo
- 414. Tsinghua University
- 415. University of Tübingen
- 416. Vanderbilt University
- 417. University of Washington
- 418. Wayne State University
- 419. University of Natural Resources and Applied Life Sciences
- 420. Julius Maximilian University of Würzburg
- 421. Yale University
- 422. Yamaguchi University
- 423. Zhejiang University
- 424. University of Cologne
- 425. University of Verona
- 426. Laval University
- 427. University of Houston System
- 428. UNUM Therapeutics
- 429. University of Victoria
- 430. UWELL BioPharma
- 431. Var2 Pharmaceuticals
- 432. Verastem Oncology
- 433. Blood Research Institute - Versiti
- 434. ViroMed
- 435. Vivia Biotech
- 436. Vor Biopharma.
- 437. Vycellix
- 438. Western Sydney Local Health District
- 439. West China hospital, sichuan university
- 440. University of Münster
- 441. Whitehead Institute
- 442. WindMIL Therapeutics
- 443. Wisconsin Alumni Research Foundation
- 444. Wilson Wolf Manufacturing
- 445. Wugen
- 446. Wuhan Bio-Raid Biotechnology Co. Ltd.
- 447. WuXi Biologics
- 448. Y-mAbs Therapeutics, Inc.
- 449. Yeda Research & Development Co Ltd
- 450. Yissum Technology Transfer Company of the Hebrew University
- 451. Shanghai Yizun Pharmaceutical Technology
- 452. Yufan Biotechnologies
- 453. zPREDICTA
- 454. Zymeworks













