 |
市場調查報告書
商品編碼
1170393
視神經脊髓炎頻譜障礙(NMOSD)市場:1次調查(KOL的洞察) - 市場情報 - 流行病學和到2032年的市場預測Neuromyelitis Optica Spectrum Disorder (NMOSD) | Primary Research (KOL's Insight) | Market Intelligence | Epidemiology & Market Forecast-2032 |
||||||
本報告提供全球視神經脊髓炎頻譜障礙(NMOSD)市場相關調查,市場現狀,以及病例數趨勢,患者趨勢,競爭產品的市場上地位,市場機會等資訊。
目錄
摘要整理
視神經脊髓炎頻譜障礙(NMOSD)疾病的背景
- 視神經脊髓炎頻譜障礙(NMOSD):主要的焦點
- 意義
- 症狀和原因
- 分類
- 病理學
- NMOSD的病理學的機制和治療標的
- 診斷
流行病學的估計和2032年為止的預測
- 主要調查結果
- 手法和資料來源
- 視神經脊髓炎頻譜障礙(NMOSD)的各國患病數量(美國,德國,法國,義大利,西班牙,英國,及日本)
- 各國視神經脊髓炎頻譜障礙(NMOSD)的急性及慢性治療數量
- 各國慢性難治性/復發性(R/R)視神經脊髓炎頻譜障礙(NMOSD)的患病數量
視神經脊髓炎頻譜障礙(NMOSD)的流行病學和模式參數的主要的資訊來源
- 美國
- 德國
- 法國
- 義大利
- 西班牙
- 英國
- 日本
目前治療方法和醫療行為
- 治療和醫療行為
- NMOSD治療流程
已上市藥
- Eculizumab
- Satralizumab
- Inebilizumab
未滿足需求
新的治療方法
- 產品分析
- Ravulizumab
- Telitacicept
- SHR1459
- BAT4406F
- Batoclimab
- XT-0528
- Ublituximab
- Lu AG06466
- Equecabtagene Autoleucel
- CRD1
視神經脊髓炎頻譜障礙(NMOSD)- 費用負擔與處方箋調查
未來的治療範例
現在及新的治療方法的年度費用
市場預測
- 主要調查結果
- 到2032年的各國市場預測
各國市場預測
- 美國
- 德國
- 法國
- 義大利
- 西班牙
- 英國
- 日本
推動市場要素與阻礙因素
We see great future treatment opportunity for the NMOSD market because of the launch of the emerging therapies like Ravulizumab (Alexion Pharmaceuticals), Telitacicept (RC-Pharma), SHR1459 (Reistone Biopharma) and other potential therapies, providing the global solution for "optic neuritis with spinal cord manifestations and other neurologic disorders associated with the serum aquaporin-4 immunoglobulin G antibodies".
Neuromyelitis Optica Spectrum Disorder (NMOSD) is an inflammatory, demyelinating, antibody-mediated disease of the central nervous system (CNS) that predominantly targets the optic nerves, brainstem, and spinal cord. The NMOSD involves aquaporin 4 (AQP4) which is a membrane-bound protein with a high concentration in a certain region of the central nervous system, such as the optic nerve, and the spinal cord which is being attacked by the IgG antibody.
Neuromyelitis Optica Spectrum Disorder (NMOSD)- Epidemiology
NMOSD can be categorized into two pathophysiological entities depending on the presence or absence of AQP4-Ab. In approx. 80% of cases, AQP4-Ab can be detected, which causes a primarily astrocytopathic disease. Interestingly, in about 50% of the AQP4-Ab seronegative NMOSD patients autoantibodies against myelin-oligodendrocyte-glycoprotein (MOG-Ab) can be seen, whose pathomorphological correlate is primarily oligodendrocytopathic.AQP4-IgG testing in cases with good clinical reason to suspect NMOSD may be of value and that a positive AQP4-IgG test lessens the clinical and radiologic requirements for a diagnosis of NMOSD; these principles guided the development of the diagnostic criteria proposed by the IPND. The 2015 diagnostic criteria outline a stratified diagnosis based on the presence or absence of serum aquaporin-4 immunoglobulin G antibodies (AQP4-IgG), which are highly specific for NMOSD. Most-though not all-patients with NMO have detectable serum antibodies that target AQP4-IgG.
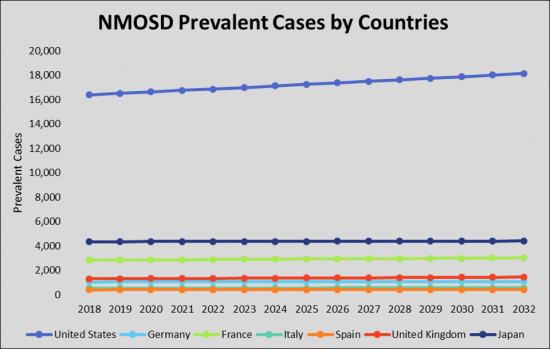
As per estimates, the United States accounted for the highest prevalence of Neuromyelitis Optica Spectrum Disorder (NMOSD) cases are expected to reach ~18,152 by 2032 during the study period.

Neuromyelitis Optica Spectrum Disorder (NMOSD)- Current Market Size & Forecast Trends
The current therapies market to treat Neuromyelitis Optica Spectrum Disorder (NMOSD) has transformed in recent years with new options for a disease that have the potential to significantly improve patient outcomes. In countries where all three of these new treatments are approved for clinical use, clinicians are now faced with a changing landscape of options to offer their patients with NMOSD, who might have different priorities among efficacy, safety, cost, monitoring burden, and logistics. The current therapies market to treat NMOSD is large as 100% of the affected population will be prescribed with immunosuppressive agents as the first line of therapy.
Currently, three medications have been approved for the treatment of NMOSD and these include satralizumab, eculizumab and inebilizumab. These medications have shown promising results concerning short-term efficacy. However, there is no data on long-term safety and efficacy in NMOSD patients and their efficacy in AQP4-Ab negative NMOSD patients is not yet known. At present, drugs such as azathioprine, rituximab and mycophenolate mofetil are being used to prevent relapse for patients with NMOSD. However, recurrent relapse in patients with NMOSD is still common which is due to the lack of specific therapeutic target. Additionally, some patients are intolerable to certain treatments due to their side effects, such as avascular necrosis of femoral head and hepatic impairment.
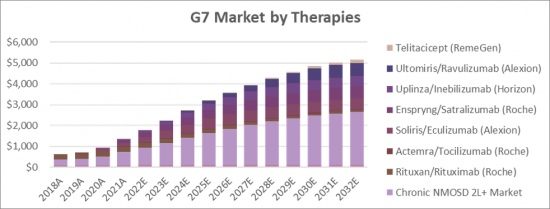
The Neuromyelitis Optica Spectrum Disorder (NMOSD) therapeutics market is expected to increase at a significant rate during the figure time frame, 2021 to 2032. In 2021, the market is developing at a consistent rate and the market is relied upon to ascend over the projected horizon with the rise in adoption strategies by key market players. The therapy market is expected to experience high growth throughout our study period, increasing from USD 362 million in 2018 to USD 2.64 billion in 2032, representing compound annual growth (CAGR) of 15.25%. Primary drivers of this growth will be the uptake of the existing drugs i.e., Soliris/Eculizumab (Alexion), Enspryng/Satralizumab (Roche), and Uplinza/Inebilizumab (Horizon) as well as the launch of the new drugs i.e., Ravulizumab (Alexion) Telitacicept (RemeGen).

Report Highlights:
- Neuromyelitis Optica Spectrum Disorder (NMOSD) - Current Market Trends
- Neuromyelitis Optica Spectrum Disorder (NMOSD) - Current & Forecasted Cases across the G7 Countries
- Neuromyelitis Optica Spectrum Disorder (NMOSD) - Market Opportunities And Sales Potential for Agents
- Neuromyelitis Optica Spectrum Disorder (NMOSD) - Patient-based Market Forecast to 2030
- Neuromyelitis Optica Spectrum Disorder (NMOSD) - Untapped Business Opportunities
- Neuromyelitis Optica Spectrum Disorder (NMOSD) - Product Positioning Vis-a-vis Competitors' Products
- Neuromyelitis Optica Spectrum Disorder (NMOSD) - KOLs Insight
Table of Contents
Executive Summary
- Key Findings
Neuromyelitis Optica Spectrum Disorder (NMOSD) Disease Background
- Neuromyelitis Optica Spectrum Disorder (NMOSD): Key Highlights
- Definition
- Symptoms & Causes
- Classifications
- Pathophysiology
- Pathophysiologic Mechanisms and Therapeutic Targets in NMOSD
- Diagnosis
Epidemiology Estimated and Forecast to 2032
- Key Findings
- Methods and Data Sources
- Country Specific Prevalent Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD) (US, Germany, France, Italy, Spain, UK, and Japan)
- Country Specific Acute & Chronic Treated Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Country Specific Chronic Refractory/Relapsed (R/R) Prevalent cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
Key Sources for Neuromyelitis Optica Spectrum Disorder (NMOSD) Epidemiology and Model Parameters
- United States
- United States Prevalent Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- United States Acute & Chronic Treated Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- United States Chronic Refractory/Relapsed (R/R) Prevalent cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Germany
- Germany Prevalent Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Germany Acute & Chronic Treated Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Germany Chronic Refractory/Relapsed (R/R) Prevalent cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- France
- France Prevalent Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- France Acute & Chronic Treated Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- France Chronic Refractory/Relapsed (R/R) Prevalent cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Italy
- Italy Prevalent Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Italy Acute & Chronic Treated Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Italy Chronic Refractory/Relapsed (R/R) Prevalent cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Spain
- Spain Prevalent Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Spain Acute & Chronic Treated Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Spain Chronic Refractory/Relapsed (R/R) Prevalent cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- United Kingdom
- United Kingdom Prevalent Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- United Kingdom Acute & Chronic Treated Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- United Kingdom Chronic Refractory/Relapsed (R/R) Prevalent cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Japan
- Japan Prevalent Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Japan Acute & Chronic Treated Cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Japan Chronic Refractory/Relapsed (R/R) Prevalent cases of Neuromyelitis Optica Spectrum Disorder (NMOSD)
Current Therapies and Medical Practice
- Treatments & Medical Practices
- NMOSD Treatment Algorithm
Marketed Drug
- Eculizumab (Alexion Pharmaceuticals)
- Satralizumab (Roche)
- Inebilizumab (Horizon Therapeutics/Viela Bio)
Unmet Needs
Emerging Therapies
- Pipeline Overview
- Therapeutic Developments Pipeline for Neuromyelitis Optica Spectrum Disorder (NMOSD)
- Product Analysis
- Ravulizumab (Alexion Pharmaceuticals)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- Telitacicept (RC-Pharma)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- SHR1459 (Reistone Biopharma)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- BAT4406F (Bio-Thera Solutions)
- Product Profile
- Clinical Development
- Sales & Market Opportunity by 2032
- Batoclimab (Harbour BioMed)
- Product Profile
- Clinical Development
- XT-0528 (Xenter Therapeutics/Arrien Pharmaceuticals/Boston Pharmaceuticals)
- Product Profile
- Clinical Development
- Ublituximab (TG Therapeutics)
- Product Profile
- Clinical Development
- Lu AG06466 (Lundbeck/Abide Therapeutics)
- Product Profile
- Clinical Development
- Equecabtagene Autoleucel (Nanjing IASO Biotherapeutic Co.,Ltd/ Innovent Biologics, Inc.)
- Product Profile
- Clinical Development
- CRD1 (Merck)
- Product Profile
- Ravulizumab (Alexion Pharmaceuticals)
- Product Analysis
Neuromyelitis Optica Spectrum Disorder (NMOSD)- Cost Burden & Prescriptions survey
Future Treatment Paradigm
- Neuromyelitis Optica Spectrum Disorder (NMOSD) Competitor Landscape and Approvals Anticipated
- Future Treatment Algorithms and Competitor Positioning
- Key Data Summary for Emerging Treatment
Annual Cost of Current & Emerging Therapies
Market Outlook
- Key Findings
- Country Specific Market Forecast to 2032
- G7 total Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) 2018-2032 (USD Million)
- G7 total Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) by Therapies 2018-2032 (USD Million)
Market Forecast by Country
- United States
- United States Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) 2018-2032 (USD Million)
- United States Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) by Therapies 2018-2032 (USD Million)
- Germany
- Germany Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) 2018-2032 (USD Million)
- Germany Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) by Therapies 2018-2032 (USD Million)
- France
- France Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) 2018-2032 (USD Million)
- France Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) by Therapies 2018-2032 (USD Million)
- Italy
- Italy Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) 2018-2032 (USD Million)
- Italy Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) by Therapies 2018-2032 (USD Million)
- Spain
- Spain Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) 2018-2032 (USD Million)
- Spain Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) by Therapies 2018-2032 (USD Million)
- United Kingdom
- United Kingdom Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) 2018-2032 (USD Million)
- United Kingdom Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) by Therapies 2018-2032 (USD Million)
- Japan
- Japan Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) 2018-2032 (USD Million)
- Japan Market for Neuromyelitis Optica Spectrum Disorder (NMOSD) by Therapies 2018-2032 (USD Million)
Market Drivers and Constraints
- What Factors Are Driving the Market for Neuromyelitis Optica Spectrum Disorder (NMOSD)?
- What Factors Are Constraining the Market for Neuromyelitis Optica Spectrum Disorder (NMOSD)?



