 |
市場調查報告書
商品編碼
1414926
免疫查核點抑制劑市場:按類型、按應用:2023-2032 年全球機會分析與產業預測Immune Checkpoint Inhibitors Market By Type, By Application : Global Opportunity Analysis and Industry Forecast, 2023-2032 |
||||||
免疫查核點抑制劑(ICI)市場2022年估值為401億美元,預計2023年至2032年複合年成長率為16.8%,到2032年達到1894億美元。
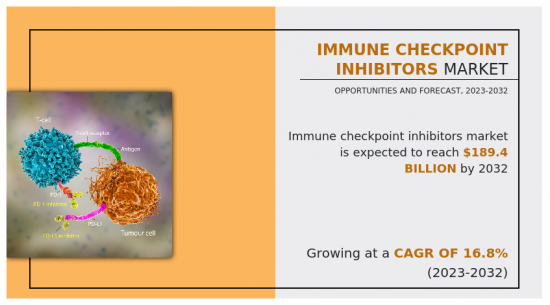
癌細胞利用這些查核點來逃避免疫系統的檢測和破壞。免疫查核點抑制劑針對特定蛋白,如PD-1(程序性細胞死亡蛋白1)、PD-L1(程序性死亡配體1)和CTLA-4(細胞毒性T淋巴球相關蛋白4),以阻斷發揮作用。透過抑制這些蛋白質,這些藥物使免疫系統能夠更有效地識別和對抗癌細胞。
推動免疫查核點抑制劑市場成長的主要因素是研發的進步、癌症患者數量的增加、臨床試驗數量的增加、研究計畫的擴大、策略舉措以及新適應症的核准擴大。癌症仍然是全世界死亡的主要原因,並且診斷出患有各種癌症類型的病例數量持續增加。例如,根據美國美國癌症研究所癌症控制和人口科學部門 (DCCPS) 的數據,美國有 623,405 人患有轉移性乳腺癌、前列腺癌、肺癌、大腸或轉移性黑色素瘤。隨著癌症負擔的增加,迫切需要更有效的治療方案,從而增加了對免疫查核點抑制劑的需求。
此外,越來越多的針對免疫查核點抑制劑的臨床試驗預計也將促進市場成長。製藥公司、生技公司和學術機構擴大將資源投入臨床研究。該投資旨在研究免疫查核點抑制劑在多種癌症類型中的安全性、有效性和可用性。這些臨床試驗的重點是探索新型藥物組合、評估治療反應和識別預測性生物標記物,為擴大我們對免疫主導療法的理解做出了重大貢獻。例如,2023 年 7 月,默克公司和 Moderna 公司宣布,他們的臨床實驗性個人化新抗原輔助性治療(宣布啟動結合V940 (mRNA-4157) 的關鍵3 期隨機V940-001 臨床試驗,V940 (mRNA-4157 ) 是一種INT 藥物) ),與默沙東的抗PD-1療法KEYTRUDA合併使用。此類臨床試驗的結果不僅可以作為藥物開拓的驅動力,還有助於確定治療策略,進一步支持免疫查核點抑制劑市場的成長。
此外,製藥公司、生技公司和學術機構之間的策略聯盟已成為推動免疫查核點抑制劑市場成長的關鍵因素。夥伴關係和聯盟促進專業知識、資源和技術的整合,以加快藥物研發和開發過程。例如,2020年5月,全球生物製藥公司信達生物與德克薩斯大學MD安德森癌症中心宣布推出信達生物抗PD-1單株抗體達伯舒(信迪利單抗注射液),使用於治療罕見癌症我們宣布,我們已簽訂戰略合作夥伴協議,在美國共同開發該藥物。透過此類合作研究,我們已成功開發並商業化了多種免疫查核點抑制劑,擴大了免疫查核點抑制劑在癌症領域的應用範圍。
目錄
第1章簡介
第 2 章執行摘要
第3章市場概況
- 市場定義和範圍
- 主要發現
- 影響因素
- 主要投資機會
- 波特五力分析
- 市場動態
- 促進因素
- 全球癌症發生率增加
- 老年人口增加
- 免疫查核點抑制劑支持報銷政策
- 抑制因素
- 免疫查核點抑制劑價格高
- 機會
- 管道藥物增加
- 新興市場的成長機會
- 促進因素
第4章免疫查核點抑制劑市場:依類型
- 概述
- CTLA-4抑制劑
- PD-1抑制劑
- PD-L1抑制劑
第5章免疫查核點抑制劑市場:依應用分類
- 概述
- 肺癌
- 膀胱癌
- 黑色素瘤
- 大腸
- 霍奇金淋巴瘤
- 其他
第6章免疫查核點抑制劑市場:按地區
- 概述
- 北美洲
- 美國
- 加拿大
- 墨西哥
- 歐洲
- 德國
- 法國
- 英國
- 其他
- 亞太地區
- 日本
- 中國
- 印度
- 澳洲
- 韓國
- 其他
- 拉丁美洲/中東/非洲
- 巴西
- 其他
第7章 競爭形勢
- 介紹
- 關鍵成功策略
- 10家主要企業產品圖譜
- 競爭對手儀表板
- 競爭熱圖
- 主要企業定位(2022年)
第8章 公司簡介
- Merck & Co., Inc.
- AstraZeneca plc
- F. Hoffmann-La Roche Ltd.
- Merck KGaA
- Regeneron Pharmaceuticals, Inc.
- Bristol-Myers Squibb Company
- BeiGene, Ltd.
- Shanghai Junshi Biosciences Co., Ltd.
- GlaxoSmithKline plc
- Innovent Biologics, Inc.
According to a new report published by Allied Market Research, titled, "Immune Checkpoint Inhibitors Market," The immune checkpoint inhibitors market was valued at $40.1 billion in 2022, and is estimated to reach $189.4 billion by 2032, growing at a CAGR of 16.8% from 2023 to 2032.

Cancer cells exploit these checkpoints to evade the immune system's detection and destruction. Immune checkpoint inhibitors work by targeting and blocking specific proteins, such as PD-1 (programmed cell death protein 1), PD-L1 (programmed death-ligand 1), or CTLA-4 (cytotoxic T-lymphocyte-associated protein 4), which are involved in these checkpoints. By inhibiting these proteins, these drugs enable the immune system to more effectively identify and combat cancer cells.
The major factors driving the growth of immune checkpoint inhibitors market are advancements in R&D, increase in prevalence of cancer cases, increase in number of clinical trials, expanding research initiatives, strategic collaborations, and growing approvals for new indications. Cancer remains a leading cause of mortality globally, with continuous rise in diagnosed cases across various cancer types. For instance, National Cancer Institute, Division of Cancer Control & Population Sciences (DCCPS), stated that, 623,405 people are living with metastatic breast, prostate, lung, colorectal cancer, or metastatic melanoma in the U.S. As the burden of cancer grows, there is a pressing need for more effective treatment options, leading to the heightened demand for immune checkpoint inhibitors.
Further, increase in the number of clinical trials focusing on the immune checkpoint inhibitors is expected to fuel the market growth. There is a growing inclination among pharmaceutical companies, biotechnology firms, and academic institutions to allocate more resources toward clinical research. This investment aims to scrutinize the safety, effectiveness, and possible uses of immune checkpoint inhibitors across diverse cancer types. These trials, focused on investigating novel drug combinations, evaluating treatment reactions, and pinpointing predictive biomarkers, significantly contribute to broadening the comprehension of immune-driven treatments. For instance, in July 2023, Merck & Co., Inc., and Moderna, Inc. announced the initiation of the pivotal Phase 3 randomized V940-001 clinical trial evaluating V940 (mRNA-4157), an investigational individualized neoantigen therapy (INT), in combination with KEYTRUDA, Merck's anti-PD-1 therapy, as an adjuvant treatment in patients with resected high-risk (Stage IIB-IV) melanoma. The results from these trials not only drive drug development but also inform treatment decisions, further boosting the growth of the immune checkpoint inhibitors market.
Furthermore, strategic collaborations among pharmaceutical companies, biotechnology firms, and academic institutions have emerged as a key factor propelling the growth of the immune checkpoint inhibitors market. Partnerships and alliances facilitate the consolidation of expertise, resources, and technologies, expediting the drug discovery and development process. For instance, in May 2020, Innovent Biologics, Inc., a world-class biopharmaceutical company that develops, manufactures, and commercializes high-quality medicines for the treatment of cancer, autoimmune, metabolic & other major diseases, and The University of Texas MD Anderson Cancer Center, announced a strategic collaboration agreement to co-develop TYVYT (sintilimab injection), Innovent's anti-PD-1 monoclonal antibody, for treating rare cancers in the U.S. In addition, collaborative efforts facilitate the exploration of diverse therapeutic applications, the conduct of larger clinical trials, and the accelerated translation of research findings into clinical applications. Such collaborations have led to the successful development and commercialization of several immune checkpoint inhibitors, widening their reach and applications in oncology.
The immune checkpoint inhibitors market is segmented on the basis of product type, application, and region. By product type, the market is categorized into CTLA-4 inhibitor, PD-1 inhibitor, and PD-L1 inhibitor. On the basis of application, the market is classified into lung cancer, bladder cancer, melanoma, colorectal cancer, Hodgkin lymphoma, and others. Region-wise, the market is analyzed across North America (the U.S., Canada, and Mexico), Europe (Germany, France, the UK, and rest of Europe), Asia-Pacific (Japan, China, Australia, India, South Korea, and rest of Asia-Pacific), and LAMEA (Brazil, and rest of LAMEA).
Major key players that operate in the global immune checkpoint inhibitors market are Merck & Co., Inc., AstraZeneca plc, F. Hoffmann-La Roche Ltd., Merck KGaA, Regeneron Pharmaceuticals, Inc., Bristol-Myers Squibb Company, BeiGene, Ltd., Shanghai Junshi Biosciences Co., Ltd., GlaxoSmithKline plc, and Innovent Biologics, Inc. The key players have adopted strategies such as acquisition, agreement, strategic alliance, collaboration, clinical trial, expansion, joint venture, new product development, and product approval to expand their product portfolio.
Key Benefits For Stakeholders
- This report provides a quantitative analysis of the market segments, current trends, estimations, and dynamics of the immune checkpoint inhibitors market analysis from 2022 to 2032 to identify the prevailing immune checkpoint inhibitors market opportunities.
- The market research is offered along with information related to key drivers, restraints, and opportunities.
- Porter's five forces analysis highlights the potency of buyers and suppliers to enable stakeholders make profit-oriented business decisions and strengthen their supplier-buyer network.
- In-depth analysis of the immune checkpoint inhibitors market segmentation assists to determine the prevailing market opportunities.
- Major countries in each region are mapped according to their revenue contribution to the global market.
- Market player positioning facilitates benchmarking and provides a clear understanding of the present position of the market players.
- The report includes the analysis of the regional as well as global immune checkpoint inhibitors market trends, key players, market segments, application areas, and market growth strategies.
Additional benefits you will get with this purchase are:
- Quarterly Update and* (only available with a corporate license, on listed price)
- 5 additional Company Profile of client Choice pre- or Post-purchase, as a free update.
- Free Upcoming Version on the Purchase of Five and Enterprise User License.
- 16 analyst hours of support* (post-purchase, if you find additional data requirements upon review of the report, you may receive support amounting to 16 analyst hours to solve questions, and post-sale queries)
- 15% Free Customization* (in case the scope or segment of the report does not match your requirements, 15% is equivalent to 3 working days of free work, applicable once)
- Free data Pack on the Five and Enterprise User License. (Excel version of the report)
- Free Updated report if the report is 6-12 months old or older.
- 24-hour priority response*
- Free Industry updates and white papers.
Possible Customization with this report (with additional cost and timeline, please talk to the sales executive to know more)
- Regulatory Guidelines
- Additional company profiles with specific to client's interest
- Additional country or region analysis- market size and forecast
- Expanded list for Company Profiles
- Historic market data
- Key player details (including location, contact details, supplier/vendor network etc. in excel format)
Key Market Segments
By Type
- CTLA-4 inhibitor
- PD-1 inhibitor
- PD-L1 inhibitor
By Application
- Lung Cancer
- Bladder Cancer
- Melanoma
- Colorectal Cancer
- Hodgkin lymphoma
- Others
By Region
- North America
- U.S.
- Canada
- Mexico
- Europe
- Germany
- France
- UK
- Rest of Europe
- Asia-Pacific
- Japan
- China
- India
- Australia
- South Korea
- Rest of Asia-Pacific
- LAMEA
- Brazil
- Rest of LAMEA
Key Market Players:
- Merck & Co., Inc.
- AstraZeneca plc
- Merck KGaA
- Bristol-Myers Squibb Company
- BeiGene, Ltd.
- Shanghai Junshi Biosciences Co., Ltd.
- GlaxoSmithKline plc
- Innovent Biologics, Inc.
- F. Hoffmann-La Roche Ltd.
- Regeneron Pharmaceuticals, Inc.
TABLE OF CONTENTS
CHAPTER 1: INTRODUCTION
- 1.1. Report description
- 1.2. Key market segments
- 1.3. Key benefits to the stakeholders
- 1.4. Research methodology
- 1.4.1. Primary research
- 1.4.2. Secondary research
- 1.4.3. Analyst tools and models
CHAPTER 2: EXECUTIVE SUMMARY
- 2.1. CXO Perspective
CHAPTER 3: MARKET OVERVIEW
- 3.1. Market definition and scope
- 3.2. Key findings
- 3.2.1. Top impacting factors
- 3.2.2. Top investment pockets
- 3.3. Porter's five forces analysis
- 3.3.1. High bargaining power of suppliers
- 3.3.2. Moderate threat of new entrants
- 3.3.3. Low threat of substitutes
- 3.3.4. Low intensity of rivalry
- 3.3.5. High bargaining power of buyers
- 3.4. Market dynamics
- 3.4.1. Drivers
- 3.4.1.1. Increase in incidences of cancer across the globe
- 3.4.1.2. Rise in geriatric population
- 3.4.1.3. Supportive reimbursement policies for immune checkpoint inhibitors
- 3.4.2. Restraints
- 3.4.2.1. Higher cost of immune checkpoint inhibitors
- 3.4.3. Opportunities
- 3.4.3.1. Increase in number of pipeline drugs
- 3.4.3.2. Growth opportunities in emerging markets
- 3.4.1. Drivers
CHAPTER 4: IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE
- 4.1. Overview
- 4.1.1. Market size and forecast
- 4.2. CTLA-4 inhibitor
- 4.2.1. Key market trends, growth factors and opportunities
- 4.2.2. Market size and forecast, by region
- 4.2.3. Market share analysis by country
- 4.3. PD-1 inhibitor
- 4.3.1. Key market trends, growth factors and opportunities
- 4.3.2. Market size and forecast, by region
- 4.3.3. Market share analysis by country
- 4.4. PD-L1 inhibitor
- 4.4.1. Key market trends, growth factors and opportunities
- 4.4.2. Market size and forecast, by region
- 4.4.3. Market share analysis by country
CHAPTER 5: IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION
- 5.1. Overview
- 5.1.1. Market size and forecast
- 5.2. Lung Cancer
- 5.2.1. Key market trends, growth factors and opportunities
- 5.2.2. Market size and forecast, by region
- 5.2.3. Market share analysis by country
- 5.3. Bladder Cancer
- 5.3.1. Key market trends, growth factors and opportunities
- 5.3.2. Market size and forecast, by region
- 5.3.3. Market share analysis by country
- 5.4. Melanoma
- 5.4.1. Key market trends, growth factors and opportunities
- 5.4.2. Market size and forecast, by region
- 5.4.3. Market share analysis by country
- 5.5. Colorectal Cancer
- 5.5.1. Key market trends, growth factors and opportunities
- 5.5.2. Market size and forecast, by region
- 5.5.3. Market share analysis by country
- 5.6. Hodgkin lymphoma
- 5.6.1. Key market trends, growth factors and opportunities
- 5.6.2. Market size and forecast, by region
- 5.6.3. Market share analysis by country
- 5.7. Others
- 5.7.1. Key market trends, growth factors and opportunities
- 5.7.2. Market size and forecast, by region
- 5.7.3. Market share analysis by country
CHAPTER 6: IMMUNE CHECKPOINT INHIBITORS MARKET, BY REGION
- 6.1. Overview
- 6.1.1. Market size and forecast By Region
- 6.2. North America
- 6.2.1. Key market trends, growth factors and opportunities
- 6.2.2. Market size and forecast, by Type
- 6.2.3. Market size and forecast, by Application
- 6.2.4. Market size and forecast, by country
- 6.2.4.1. U.S.
- 6.2.4.1.1. Market size and forecast, by Type
- 6.2.4.1.2. Market size and forecast, by Application
- 6.2.4.2. Canada
- 6.2.4.2.1. Market size and forecast, by Type
- 6.2.4.2.2. Market size and forecast, by Application
- 6.2.4.3. Mexico
- 6.2.4.3.1. Market size and forecast, by Type
- 6.2.4.3.2. Market size and forecast, by Application
- 6.3. Europe
- 6.3.1. Key market trends, growth factors and opportunities
- 6.3.2. Market size and forecast, by Type
- 6.3.3. Market size and forecast, by Application
- 6.3.4. Market size and forecast, by country
- 6.3.4.1. Germany
- 6.3.4.1.1. Market size and forecast, by Type
- 6.3.4.1.2. Market size and forecast, by Application
- 6.3.4.2. France
- 6.3.4.2.1. Market size and forecast, by Type
- 6.3.4.2.2. Market size and forecast, by Application
- 6.3.4.3. UK
- 6.3.4.3.1. Market size and forecast, by Type
- 6.3.4.3.2. Market size and forecast, by Application
- 6.3.4.4. Rest of Europe
- 6.3.4.4.1. Market size and forecast, by Type
- 6.3.4.4.2. Market size and forecast, by Application
- 6.4. Asia-Pacific
- 6.4.1. Key market trends, growth factors and opportunities
- 6.4.2. Market size and forecast, by Type
- 6.4.3. Market size and forecast, by Application
- 6.4.4. Market size and forecast, by country
- 6.4.4.1. Japan
- 6.4.4.1.1. Market size and forecast, by Type
- 6.4.4.1.2. Market size and forecast, by Application
- 6.4.4.2. China
- 6.4.4.2.1. Market size and forecast, by Type
- 6.4.4.2.2. Market size and forecast, by Application
- 6.4.4.3. India
- 6.4.4.3.1. Market size and forecast, by Type
- 6.4.4.3.2. Market size and forecast, by Application
- 6.4.4.4. Australia
- 6.4.4.4.1. Market size and forecast, by Type
- 6.4.4.4.2. Market size and forecast, by Application
- 6.4.4.5. South Korea
- 6.4.4.5.1. Market size and forecast, by Type
- 6.4.4.5.2. Market size and forecast, by Application
- 6.4.4.6. Rest of Asia-Pacific
- 6.4.4.6.1. Market size and forecast, by Type
- 6.4.4.6.2. Market size and forecast, by Application
- 6.5. LAMEA
- 6.5.1. Key market trends, growth factors and opportunities
- 6.5.2. Market size and forecast, by Type
- 6.5.3. Market size and forecast, by Application
- 6.5.4. Market size and forecast, by country
- 6.5.4.1. Brazil
- 6.5.4.1.1. Market size and forecast, by Type
- 6.5.4.1.2. Market size and forecast, by Application
- 6.5.4.2. Rest of LAMEA
- 6.5.4.2.1. Market size and forecast, by Type
- 6.5.4.2.2. Market size and forecast, by Application
CHAPTER 7: COMPETITIVE LANDSCAPE
- 7.1. Introduction
- 7.2. Top winning strategies
- 7.3. Product mapping of top 10 player
- 7.4. Competitive dashboard
- 7.5. Competitive heatmap
- 7.6. Top player positioning, 2022
CHAPTER 8: COMPANY PROFILES
- 8.1. Merck & Co., Inc.
- 8.1.1. Company overview
- 8.1.2. Key executives
- 8.1.3. Company snapshot
- 8.1.4. Operating business segments
- 8.1.5. Product portfolio
- 8.1.6. Business performance
- 8.1.7. Key strategic moves and developments
- 8.2. AstraZeneca plc
- 8.2.1. Company overview
- 8.2.2. Key executives
- 8.2.3. Company snapshot
- 8.2.4. Operating business segments
- 8.2.5. Product portfolio
- 8.2.6. Business performance
- 8.2.7. Key strategic moves and developments
- 8.3. F. Hoffmann-La Roche Ltd.
- 8.3.1. Company overview
- 8.3.2. Key executives
- 8.3.3. Company snapshot
- 8.3.4. Operating business segments
- 8.3.5. Product portfolio
- 8.3.6. Business performance
- 8.3.7. Key strategic moves and developments
- 8.4. Merck KGaA
- 8.4.1. Company overview
- 8.4.2. Key executives
- 8.4.3. Company snapshot
- 8.4.4. Operating business segments
- 8.4.5. Product portfolio
- 8.4.6. Business performance
- 8.4.7. Key strategic moves and developments
- 8.5. Regeneron Pharmaceuticals, Inc.
- 8.5.1. Company overview
- 8.5.2. Key executives
- 8.5.3. Company snapshot
- 8.5.4. Operating business segments
- 8.5.5. Product portfolio
- 8.5.6. Business performance
- 8.5.7. Key strategic moves and developments
- 8.6. Bristol-Myers Squibb Company
- 8.6.1. Company overview
- 8.6.2. Key executives
- 8.6.3. Company snapshot
- 8.6.4. Operating business segments
- 8.6.5. Product portfolio
- 8.6.6. Business performance
- 8.6.7. Key strategic moves and developments
- 8.7. BeiGene, Ltd.
- 8.7.1. Company overview
- 8.7.2. Key executives
- 8.7.3. Company snapshot
- 8.7.4. Operating business segments
- 8.7.5. Product portfolio
- 8.7.6. Business performance
- 8.7.7. Key strategic moves and developments
- 8.8. Shanghai Junshi Biosciences Co., Ltd.
- 8.8.1. Company overview
- 8.8.2. Key executives
- 8.8.3. Company snapshot
- 8.8.4. Operating business segments
- 8.8.5. Product portfolio
- 8.8.6. Business performance
- 8.8.7. Key strategic moves and developments
- 8.9. GlaxoSmithKline plc
- 8.9.1. Company overview
- 8.9.2. Key executives
- 8.9.3. Company snapshot
- 8.9.4. Operating business segments
- 8.9.5. Product portfolio
- 8.9.6. Business performance
- 8.9.7. Key strategic moves and developments
- 8.10. Innovent Biologics, Inc.
- 8.10.1. Company overview
- 8.10.2. Key executives
- 8.10.3. Company snapshot
- 8.10.4. Operating business segments
- 8.10.5. Product portfolio
- 8.10.6. Business performance
- 8.10.7. Key strategic moves and developments
LIST OF TABLES
- TABLE 01. GLOBAL IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 02. IMMUNE CHECKPOINT INHIBITORS MARKET FOR CTLA-4 INHIBITOR, BY REGION, 2022-2032 ($MILLION)
- TABLE 03. IMMUNE CHECKPOINT INHIBITORS MARKET FOR PD-1 INHIBITOR, BY REGION, 2022-2032 ($MILLION)
- TABLE 04. IMMUNE CHECKPOINT INHIBITORS MARKET FOR PD-L1 INHIBITOR, BY REGION, 2022-2032 ($MILLION)
- TABLE 05. GLOBAL IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 06. IMMUNE CHECKPOINT INHIBITORS MARKET FOR LUNG CANCER, BY REGION, 2022-2032 ($MILLION)
- TABLE 07. IMMUNE CHECKPOINT INHIBITORS MARKET FOR BLADDER CANCER, BY REGION, 2022-2032 ($MILLION)
- TABLE 08. IMMUNE CHECKPOINT INHIBITORS MARKET FOR MELANOMA, BY REGION, 2022-2032 ($MILLION)
- TABLE 09. IMMUNE CHECKPOINT INHIBITORS MARKET FOR COLORECTAL CANCER, BY REGION, 2022-2032 ($MILLION)
- TABLE 10. IMMUNE CHECKPOINT INHIBITORS MARKET FOR HODGKIN LYMPHOMA, BY REGION, 2022-2032 ($MILLION)
- TABLE 11. IMMUNE CHECKPOINT INHIBITORS MARKET FOR OTHERS, BY REGION, 2022-2032 ($MILLION)
- TABLE 12. IMMUNE CHECKPOINT INHIBITORS MARKET, BY REGION, 2022-2032 ($MILLION)
- TABLE 13. NORTH AMERICA IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 14. NORTH AMERICA IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 15. NORTH AMERICA IMMUNE CHECKPOINT INHIBITORS MARKET, BY COUNTRY, 2022-2032 ($MILLION)
- TABLE 16. U.S. IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 17. U.S. IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 18. CANADA IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 19. CANADA IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 20. MEXICO IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 21. MEXICO IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 22. EUROPE IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 23. EUROPE IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 24. EUROPE IMMUNE CHECKPOINT INHIBITORS MARKET, BY COUNTRY, 2022-2032 ($MILLION)
- TABLE 25. GERMANY IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 26. GERMANY IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 27. FRANCE IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 28. FRANCE IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 29. UK IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 30. UK IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 31. REST OF EUROPE IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 32. REST OF EUROPE IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 33. ASIA-PACIFIC IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 34. ASIA-PACIFIC IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 35. ASIA-PACIFIC IMMUNE CHECKPOINT INHIBITORS MARKET, BY COUNTRY, 2022-2032 ($MILLION)
- TABLE 36. JAPAN IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 37. JAPAN IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 38. CHINA IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 39. CHINA IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 40. INDIA IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 41. INDIA IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 42. AUSTRALIA IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 43. AUSTRALIA IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 44. SOUTH KOREA IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 45. SOUTH KOREA IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 46. REST OF ASIA-PACIFIC IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 47. REST OF ASIA-PACIFIC IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 48. LAMEA IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 49. LAMEA IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 50. LAMEA IMMUNE CHECKPOINT INHIBITORS MARKET, BY COUNTRY, 2022-2032 ($MILLION)
- TABLE 51. BRAZIL IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 52. BRAZIL IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 53. REST OF LAMEA IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022-2032 ($MILLION)
- TABLE 54. REST OF LAMEA IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022-2032 ($MILLION)
- TABLE 55. MERCK & CO., INC.: KEY EXECUTIVES
- TABLE 56. MERCK & CO., INC.: COMPANY SNAPSHOT
- TABLE 57. MERCK & CO., INC.: PRODUCT SEGMENTS
- TABLE 58. MERCK & CO., INC.: PRODUCT PORTFOLIO
- TABLE 59. MERCK & CO., INC.: KEY STRATERGIES
- TABLE 60. ASTRAZENECA PLC: KEY EXECUTIVES
- TABLE 61. ASTRAZENECA PLC: COMPANY SNAPSHOT
- TABLE 62. ASTRAZENECA PLC: PRODUCT SEGMENTS
- TABLE 63. ASTRAZENECA PLC: PRODUCT PORTFOLIO
- TABLE 64. ASTRAZENECA PLC: KEY STRATERGIES
- TABLE 65. F. HOFFMANN-LA ROCHE LTD.: KEY EXECUTIVES
- TABLE 66. F. HOFFMANN-LA ROCHE LTD.: COMPANY SNAPSHOT
- TABLE 67. F. HOFFMANN-LA ROCHE LTD.: PRODUCT SEGMENTS
- TABLE 68. F. HOFFMANN-LA ROCHE LTD.: PRODUCT PORTFOLIO
- TABLE 69. F. HOFFMANN-LA ROCHE LTD.: KEY STRATERGIES
- TABLE 70. MERCK KGAA: KEY EXECUTIVES
- TABLE 71. MERCK KGAA: COMPANY SNAPSHOT
- TABLE 72. MERCK KGAA: PRODUCT SEGMENTS
- TABLE 73. MERCK KGAA: PRODUCT PORTFOLIO
- TABLE 74. MERCK KGAA: KEY STRATERGIES
- TABLE 75. REGENERON PHARMACEUTICALS, INC.: KEY EXECUTIVES
- TABLE 76. REGENERON PHARMACEUTICALS, INC.: COMPANY SNAPSHOT
- TABLE 77. REGENERON PHARMACEUTICALS, INC.: PRODUCT SEGMENTS
- TABLE 78. REGENERON PHARMACEUTICALS, INC.: PRODUCT PORTFOLIO
- TABLE 79. REGENERON PHARMACEUTICALS, INC.: KEY STRATERGIES
- TABLE 80. BRISTOL-MYERS SQUIBB COMPANY: KEY EXECUTIVES
- TABLE 81. BRISTOL-MYERS SQUIBB COMPANY: COMPANY SNAPSHOT
- TABLE 82. BRISTOL-MYERS SQUIBB COMPANY: PRODUCT SEGMENTS
- TABLE 83. BRISTOL-MYERS SQUIBB COMPANY: PRODUCT PORTFOLIO
- TABLE 84. BRISTOL-MYERS SQUIBB COMPANY: KEY STRATERGIES
- TABLE 85. BEIGENE, LTD.: KEY EXECUTIVES
- TABLE 86. BEIGENE, LTD.: COMPANY SNAPSHOT
- TABLE 87. BEIGENE, LTD.: PRODUCT SEGMENTS
- TABLE 88. BEIGENE, LTD.: PRODUCT PORTFOLIO
- TABLE 89. BEIGENE, LTD.: KEY STRATERGIES
- TABLE 90. SHANGHAI JUNSHI BIOSCIENCES CO., LTD.: KEY EXECUTIVES
- TABLE 91. SHANGHAI JUNSHI BIOSCIENCES CO., LTD.: COMPANY SNAPSHOT
- TABLE 92. SHANGHAI JUNSHI BIOSCIENCES CO., LTD.: PRODUCT SEGMENTS
- TABLE 93. SHANGHAI JUNSHI BIOSCIENCES CO., LTD.: PRODUCT PORTFOLIO
- TABLE 94. SHANGHAI JUNSHI BIOSCIENCES CO., LTD.: KEY STRATERGIES
- TABLE 95. GLAXOSMITHKLINE PLC: KEY EXECUTIVES
- TABLE 96. GLAXOSMITHKLINE PLC: COMPANY SNAPSHOT
- TABLE 97. GLAXOSMITHKLINE PLC: PRODUCT SEGMENTS
- TABLE 98. GLAXOSMITHKLINE PLC: PRODUCT PORTFOLIO
- TABLE 99. GLAXOSMITHKLINE PLC: KEY STRATERGIES
- TABLE 100. INNOVENT BIOLOGICS, INC.: KEY EXECUTIVES
- TABLE 101. INNOVENT BIOLOGICS, INC.: COMPANY SNAPSHOT
- TABLE 102. INNOVENT BIOLOGICS, INC.: PRODUCT SEGMENTS
- TABLE 103. INNOVENT BIOLOGICS, INC.: PRODUCT PORTFOLIO
- TABLE 104. INNOVENT BIOLOGICS, INC.: KEY STRATERGIES
LIST OF FIGURES
- FIGURE 01. IMMUNE CHECKPOINT INHIBITORS MARKET, 2022-2032
- FIGURE 02. SEGMENTATION OF IMMUNE CHECKPOINT INHIBITORS MARKET,2022-2032
- FIGURE 03. TOP IMPACTING FACTORS IN IMMUNE CHECKPOINT INHIBITORS MARKET (2022 TO 2032)
- FIGURE 04. TOP INVESTMENT POCKETS IN IMMUNE CHECKPOINT INHIBITORS MARKET (2023-2032)
- FIGURE 05. HIGH BARGAINING POWER OF SUPPLIERS
- FIGURE 06. MODERATE THREAT OF NEW ENTRANTS
- FIGURE 07. LOW THREAT OF SUBSTITUTES
- FIGURE 08. LOW INTENSITY OF RIVALRY
- FIGURE 09. HIGH BARGAINING POWER OF BUYERS
- FIGURE 10. GLOBAL IMMUNE CHECKPOINT INHIBITORS MARKET:DRIVERS, RESTRAINTS AND OPPORTUNITIES
- FIGURE 11. IMMUNE CHECKPOINT INHIBITORS MARKET, BY TYPE, 2022 AND 2032(%)
- FIGURE 12. COMPARATIVE SHARE ANALYSIS OF IMMUNE CHECKPOINT INHIBITORS MARKET FOR CTLA-4 INHIBITOR, BY COUNTRY 2022 AND 2032(%)
- FIGURE 13. COMPARATIVE SHARE ANALYSIS OF IMMUNE CHECKPOINT INHIBITORS MARKET FOR PD-1 INHIBITOR, BY COUNTRY 2022 AND 2032(%)
- FIGURE 14. COMPARATIVE SHARE ANALYSIS OF IMMUNE CHECKPOINT INHIBITORS MARKET FOR PD-L1 INHIBITOR, BY COUNTRY 2022 AND 2032(%)
- FIGURE 15. IMMUNE CHECKPOINT INHIBITORS MARKET, BY APPLICATION, 2022 AND 2032(%)
- FIGURE 16. COMPARATIVE SHARE ANALYSIS OF IMMUNE CHECKPOINT INHIBITORS MARKET FOR LUNG CANCER, BY COUNTRY 2022 AND 2032(%)
- FIGURE 17. COMPARATIVE SHARE ANALYSIS OF IMMUNE CHECKPOINT INHIBITORS MARKET FOR BLADDER CANCER, BY COUNTRY 2022 AND 2032(%)
- FIGURE 18. COMPARATIVE SHARE ANALYSIS OF IMMUNE CHECKPOINT INHIBITORS MARKET FOR MELANOMA, BY COUNTRY 2022 AND 2032(%)
- FIGURE 19. COMPARATIVE SHARE ANALYSIS OF IMMUNE CHECKPOINT INHIBITORS MARKET FOR COLORECTAL CANCER, BY COUNTRY 2022 AND 2032(%)
- FIGURE 20. COMPARATIVE SHARE ANALYSIS OF IMMUNE CHECKPOINT INHIBITORS MARKET FOR HODGKIN LYMPHOMA, BY COUNTRY 2022 AND 2032(%)
- FIGURE 21. COMPARATIVE SHARE ANALYSIS OF IMMUNE CHECKPOINT INHIBITORS MARKET FOR OTHERS, BY COUNTRY 2022 AND 2032(%)
- FIGURE 22. IMMUNE CHECKPOINT INHIBITORS MARKET BY REGION, 2022 AND 2032(%)
- FIGURE 23. U.S. IMMUNE CHECKPOINT INHIBITORS MARKET, 2022-2032 ($MILLION)
- FIGURE 24. CANADA IMMUNE CHECKPOINT INHIBITORS MARKET, 2022-2032 ($MILLION)
- FIGURE 25. MEXICO IMMUNE CHECKPOINT INHIBITORS MARKET, 2022-2032 ($MILLION)
- FIGURE 26. GERMANY IMMUNE CHECKPOINT INHIBITORS MARKET, 2022-2032 ($MILLION)
- FIGURE 27. FRANCE IMMUNE CHECKPOINT INHIBITORS MARKET, 2022-2032 ($MILLION)
- FIGURE 28. UK IMMUNE CHECKPOINT INHIBITORS MARKET, 2022-2032 ($MILLION)
- FIGURE 29. REST OF EUROPE IMMUNE CHECKPOINT INHIBITORS MARKET, 2022-2032 ($MILLION)
- FIGURE 30. JAPAN IMMUNE CHECKPOINT INHIBITORS MARKET, 2022-2032 ($MILLION)
- FIGURE 31. CHINA IMMUNE CHECKPOINT INHIBITORS MARKET, 2022-2032 ($MILLION)
- FIGURE 32. INDIA IMMUNE CHECKPOINT INHIBITORS MARKET, 2022-2032 ($MILLION)
- FIGURE 33. AUSTRALIA IMMUNE CHECKPOINT INHIBITORS MARKET, 2022-2032 ($MILLION)
- FIGURE 34. SOUTH KOREA IMMUNE CHECKPOINT INHIBITORS MARKET, 2022-2032 ($MILLION)
- FIGURE 35. REST OF ASIA-PACIFIC IMMUNE CHECKPOINT INHIBITORS MARKET, 2022-2032 ($MILLION)
- FIGURE 36. BRAZIL IMMUNE CHECKPOINT INHIBITORS MARKET, 2022-2032 ($MILLION)
- FIGURE 37. REST OF LAMEA IMMUNE CHECKPOINT INHIBITORS MARKET, 2022-2032 ($MILLION)
- FIGURE 38. TOP WINNING STRATEGIES, BY YEAR (2019-2023)
- FIGURE 39. TOP WINNING STRATEGIES, BY DEVELOPMENT (2019-2023)
- FIGURE 40. TOP WINNING STRATEGIES, BY COMPANY (2019-2023)
- FIGURE 41. PRODUCT MAPPING OF TOP 10 PLAYERS
- FIGURE 42. COMPETITIVE DASHBOARD
- FIGURE 43. COMPETITIVE HEATMAP: IMMUNE CHECKPOINT INHIBITORS MARKET
- FIGURE 44. TOP PLAYER POSITIONING, 2022
- FIGURE 45. MERCK & CO., INC.: NET REVENUE, 2020-2022 ($MILLION)
- FIGURE 46. MERCK & CO., INC.: REVENUE SHARE BY SEGMENT, 2022 (%)
- FIGURE 47. MERCK & CO., INC.: REVENUE SHARE BY REGION, 2022 (%)
- FIGURE 48. ASTRAZENECA PLC: NET SALES, 2020-2022 ($MILLION)
- FIGURE 49. ASTRAZENECA PLC: REVENUE SHARE BY REGION, 2022 (%)
- FIGURE 50. F. HOFFMANN-LA ROCHE LTD.: SALES REVENUE, 2020-2022 ($MILLION)
- FIGURE 51. F. HOFFMANN-LA ROCHE LTD.: REVENUE SHARE BY SEGMENT, 2022 (%)
- FIGURE 52. MERCK KGAA: NET SALES, 2020-2022 ($MILLION)
- FIGURE 53. MERCK KGAA: REVENUE SHARE BY SEGMENT, 2022 (%)
- FIGURE 54. MERCK KGAA: REVENUE SHARE BY REGION, 2022 (%)
- FIGURE 55. REGENERON PHARMACEUTICALS, INC.: NET REVENUE, 2020-2022 ($MILLION)
- FIGURE 56. BRISTOL-MYERS SQUIBB COMPANY: NET SALES, 2020-2022 ($MILLION)
- FIGURE 57. BRISTOL-MYERS SQUIBB COMPANY: REVENUE SHARE BY REGION, 2022 (%)
- FIGURE 58. BEIGENE, LTD.: NET REVENUE, 2020-2022 ($MILLION)
- FIGURE 59. BEIGENE, LTD.: REVENUE SHARE BY REGION, 2022 (%)
- FIGURE 60. SHANGHAI JUNSHI BIOSCIENCES CO., LTD.: REVENUE SHARE BY REGION, 2022 (%)
- FIGURE 61. GLAXOSMITHKLINE PLC: NET SALES, 2020-2022 ($MILLION)
- FIGURE 62. GLAXOSMITHKLINE PLC: REVENUE SHARE BY SEGMENT, 2022 (%)
- FIGURE 63. GLAXOSMITHKLINE PLC: REVENUE SHARE BY REGION, 2022 (%)
- FIGURE 64. INNOVENT BIOLOGICS, INC.: NET REVENUE, 2019-2021 ($MILLION)
- FIGURE 65. INNOVENT BIOLOGICS, INC.: REVENUE SHARE BY REGION, 2021 (%)







