|
市場調查報告書
商品編碼
1073202
預充注射器市場(第6版):按治療領域(血液疾病,傳染病,代謝疾病,自身免疫性疾病,腫瘤疾病,神經系統疾病,眼部疾病等)Prefilled Syringes Market (6th Edition) by Therapeutic Area (Blood disorders, Infectious diseases, Metabolic disorders, Autoimmune disorders, Oncological disorders, Neurological disorders, Ophthalmic disorders and Other disorders), |
||||||
按地區來看全球預充式註射器市場,我們看到新興國家的需求增加,尤其是金磚國家(巴西、俄羅斯、印度、中國、南非)。在北非和中東的類似情況下,預充式註射器的增長率預計為 10-18%。
本報告分析了全球預充式註射器市場、產品特點和結構/材料、主要產品列表、主要製造商概況、近期技術發展/專利申請動向以及整體市場規模。我們將為您提供以下信息:趨勢展望,按治療區域、注射器類型、材料、針頭系統、注射器室數量、地區和主要市場促進/抑制因素的詳細趨勢。
文本中的圖表示例
目錄
第一章介紹
第2章執行摘要
第三章介紹
第 4 章預充式註射器:市場概述
- 分析總結
- 預填充注射器:可用/正在開發的設備列表
- 預填充注射器:開發人員列表
- 預充式註射器相關技術開發
第五章產品競爭力分析
- 分析總結
- 假設和關鍵參數
- 調查方法
- 產品競爭力分析:預充式註射器
- 玻璃筒預充式註射器
- 塑料桶預充式註射器
第 6 章預填充注射器製造商
- 分析總結
- 北美預充式註射器製造商
- Becton Dickinson
- Pfizer Hospital
- West Pharmaceutical
- 歐洲主要預充式註射器製造商
- Gerresheimer
- Ompi
- Schott
- 亞洲主要預充式註射器製造商
- Nipro (MGlas收購)
- Taisei Kako
- 其他製造商
- Arte
- J.O. Pharma (Otsuka Holdings子公司)
- Shandong Pharmaceutical Glass
- Shandong Zibo Minkang Pharmaceutical packing
- Terumo
- Vetter Pharma
- WEGO Prefills Pharmaceutical Packaging
第7章針刺傷
- 分析總結
- 發病率和相關經濟負擔
- 預防針刺傷的政府立法
- 最新預充式註射器採用的安全機制
第8章預充注射器規定
第9章預充式註射器組合產品:市場概況
- 分析總結
- 預充式註射器組合產品:獲批藥品清單
- 預充式註射器組合產品:臨床試驗藥物清單
- 預充式註射器組合產品:開發人員列表
- 預充式註射器形式的主要藥物
- 以預填充注射器形式提供的其他藥劑
- 預裝注射器 市面上流行藥物:案例研究
- HUMIRA (R)、艾伯維/衛材
- Enbrel (R)、安進/輝瑞/武田
第10章主要治療領域
- 分析總結
- 自身免疫性疾病
- 傳染病
- 神經紊亂
- 代謝紊亂
第11章預充注射器:候選藥物和合作夥伴分析的可能性
- 分析總結
- 潛在候選藥物
- 已上市的候選人
- 臨床試驗中的候選藥物
- 潛在戰略合作夥伴
第12章主要製藥公司的舉措:預充注射器組合產品
- 分析總結
- 調查方法
- 主要製藥公司
第13章近期趨勢
- 分析總結
- 與預充式註射器相關的近期舉措清單(2019-2021 年)
- 年度舉措分析
- 按倡議類型分析
- 按年份和類型分析舉措
- 地區分析
- 最活躍的公司:按計劃數量分佈
- 公司分析:按實例類型
第14章專利分析
- 分析總結
- 專利解析:預充式註射器
第15章特殊預充注射器
- 分析總結
- 預裝沖洗注射器
- 預裝稀釋注射器
- 造影劑預充式註射器
第16章與預填充注射器相關的技術進步
- 分析總結
- 凍乾預充式註射器
- 眼科預充式註射器
- 真皮填充劑預充式註射器
- 帶隔氧層的多層塑料預充式註射器
- 顆粒形成風險低的預充式註射器
- 預充式註射器潤滑技術
- 預充式註射器最終滅菌的進展
- 協助患者和醫療保健提供者使用預充式註射器
第 17 章市場規模和機會分析
- 分析總結
- 調查範圍和方法
- 全球預充式註射器市場(2022-2035)
- 預充式註射器市場:按治療區域分析
- 預充式註射器市場:注射器筒材料分析
- 預充式註射器市場:按腔室系統類型分析
- 預充式註射器市場:藥物分子類型分析
- 預充式註射器市場:區域分析
- 預充式註射器市場:特殊注射器分析
- 自身免疫性疾病預充式註射器市場(2022-2035)
- 傳染病預充式註射器市場(2022-2035)
- 用於神經系統疾病的預裝注射器市場(2022-2035)
- 血液病預充注射器市場 (2022-2035)
- 用於腫瘤疾病的預裝注射器市場(2022-2035)
- 用於代謝紊亂的預填充注射器市場 (2022-2035)
- 呼吸系統疾病預充式註射器市場(2022-2035)
- 眼病預充注射器市場(2022-2035)
- 其他症狀的預填充注射器市場(2022-2035)
第18章主要增長促進劑
- 分析總結
- 慢性病發病率增加
- 對自我注射的偏好增加
- 患者人口結構的變化
- 生物製藥/生物類似藥市場的增長
- 製藥戰略的變化
- 對預防針刺傷的興趣日益濃厚
- 自動注射器和筆式註射器的預充式註射器
第19章SWOT分析
第 20 章預填充注射器組件製造商
- 分析總結
- 元器件製造商名單
- 西藥
- 公司簡介
- 財務表現
- 產品組合
- 近期趨勢和未來前景
- Datwyler Sealing Solutions (Datwyler Group的一部分)
- Aptar Pharma (AptarGroup的一部分)
- Becton Dickinson
- Ompi (Stevanato Group的一部分)
- Lonstroff (Sumitomo Rubber Industries的一部分)
第21章預充注射器灌裝/整理服務商
- 分析總結
- 灌裝/整理預灌裝注射器
- 灌裝/整理工作外包
- 增長注意事項
- 預填充注射器:填充/完成服務提供商列表
- 成立年份分析
- 按藥物分子類型和總部位置分析
- 按經營規模分析
第22章案例研究:自動注射器
- 分析總結
- 自動注射器市場:概述
- 主要公司
- Elcam Medical (E3D Elcam Drug Delivery Devices)
- Nuance Designs
- Owen Mumford
- Scandinavian Health Limited (SHL) Group
- Union Medico
- Ypsomed
第23章結論
第24章採訪總結
- 分析總結
- Oval Medical Technologies
- Intas Pharmaceuticals
- IDT Biologika
- West Pharmaceutical
- Lonstroff
- IDEO
- 小型醫療器械公司
第25章附錄1:聚合數據
第26章附錄2:公司名單
Title:
Prefilled Syringes Market (6th Edition)
by Therapeutic Area (Blood disorders, Infectious diseases, Metabolic disorders, Autoimmune disorders, Oncological disorders, Neurological disorders, Ophthalmic disorders and Other disorders), Type of Syringe (Specialty Syringes and Others) , Type of Material (Glass and Plastic), Type of Molecule (Antibody, Peptide, Protein, Small Molecule and Vaccine), and Key Geographical Regions (North America, Europe, Asia-Pacific, Middle East and Africa and Rest of the World): Industry Trends and Global Forecasts, 2022-2035.
Example Insights:

Overview
"We are seeing an increased demand in the emerging markets, particularly within the BRICS nations, namely Brazil, Russia, India, China and South Africa. North Africa and the Middle East also present a similar scenario, characterized by a growth rate of 10-18% for prefilled syringes" - Marketing Director, a large-sized company.
According to the World Health Organization (WHO), the prevalence of chronic diseases, such as heart diseases, cancers and diabetes, has increased at a steady pace in recent years. It is worth highlighting that six out of ten individuals in the US are anticipated to be suffering from at least one chronic disease. Specifically, such patients are required to take medication on a daily basis, necessitating frequent hospital visits. As per a recent study conducted by the US Centers for Disease Control (CDC), the annual expense incurred by patients suffering from such disease conditions has increased at a significant rate and is estimated to be USD 3 trillion. It is also worth highlighting that, with the onset of COVID-19 pandemic and the associated restrictions, patients are not able to visit hospitals. In order to mitigate the aforementioned challenges, companies engaged in the pharmaceutical domain have developed self-administrable dosage forms to ease out the overall treatment process. In this context, it is important to mention that the rise in global population and shortage of healthcare facilities has paved the way for a wider adoption of self-administrable dosage forms, such as prefilled syringes, among the patients.
Prefilled syringes are regarded as one of the safest options amongst the available alternatives for the intravenous mode of administration, as they demonstrate increased ease of self-administration due to elimination of dosing errors, as well as minimize the time requirements. Further, these syringes serve as a primary drug container for various drug delivery systems, such as autoinjectors and pen-injectors. Presently, more than 110 drugs, in combination with prefilled syringes, are commercially available. Amongst these, close to 60% of the products target autoimmune disorders. However, the increase in demand for such self-administration solutions has led to more stringent safety regulations, resulting in an evident rise in the cost of safety systems / end products. To combat this challenge, many players are actively undertaking research initiatives focused on developing low-cost alternatives that can boost the penetration rate and uptake of prefilled syringes by the end users. We believe that the aforementioned developments are likely to fuel the ongoing innovation and growth of the prefilled syringes market in the foreseen future.
Scope of the Report
The "Prefilled Syringes Market (6th Edition) by Therapeutic Area (Blood disorders, Infectious diseases, Metabolic disorders, Autoimmune disorders, Oncological disorders, Neurological disorders, Ophthalmic disorders and Other disorders), Type of Syringe (Specialty Syringes and Others) , Type of Material (Glass and Plastic), Type of Molecule (Antibody, Peptide, Protein, Small Molecule and Vaccine), and Key Geographical Regions (North America, Europe, Asia-Pacific, Middle East and Africa and Rest of the World): Industry Trends and Global Forecasts, 2022-2035" report features an extensive study of the current market landscape and the likely future potential associated with the prefilled syringe market, over the next decade. The study also includes an in-depth analysis, highlighting the capabilities of various stakeholders engaged in this domain. In addition to other elements, the report features:
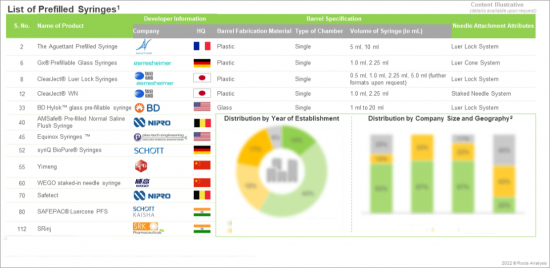
- A detailed overview of the current market landscape of players engaged in the manufacturing of prefilled syringes, based on several relevant parameters, such as year of establishment, company size, geographical location of the headquarters and manufacturing facilities, type of material used (glass and plastic), number of barrel chambers (single chamber and dual chamber), type of needle system (fixed needle system, luer lock and luer cone) and syringe volume.
- An overview of the current market landscape of companies engaged in the development of drugs in prefilled syringe format, based on several relevant parameters, such as year of establishment, company size, location of headquarters, target therapeutic area, phase of development, type of drug molecule, route of administration, approval year (for marketed products), dose strength (for marketed products) and other approved dosage forms (for marketed products).
- A detailed product competitiveness analysis of prefilled syringes, based on supplier power (in terms of employee count and annual revenues) and key product specifications (including number of chambers and needle systems, volume of the barrel and other distinguishing features).
- An in-depth analysis of marketed drugs / therapies and pipeline candidates that are likely to be available in prefilled syringe format in the near future, based on several relevant parameters, such as route of administration, type of drug molecule, target indication, other available dosage forms (for approved drugs) and historical annual sales information (for approved drugs).
- A list of nearly 80 drug developers that are likely to partner with prefilled syringe manufacturers (with regard to likely collaboration opportunities for combination product development). These players have been shortlisted based on various parameters, such as pipeline strength, target therapeutic indication(s) and developer strength.
- A review of the various prefilled syringe combination product-related initiatives undertaken by big pharma players (shortlisted on the basis of revenues generated in 2020), based on various parameters, such as current adoption (based on number of approved and under development prefilled syringe combination products) and target therapeutic area(s) and type of drug molecule.
- An analysis highlighting the key opinion leaders (KOLs) in this domain. It features a 2x2 analysis to assess the relative experience of certain KOLs, who were shortlisted based on their contributions (in terms of involvement in various trial studies, number of citations, number of publications and geographical location) in this field.
- An in-depth analysis of patents related to prefilled syringes, filed / granted, since 2015, based on several relevant parameters, such as type of patent (granted patents and patent applications), publication year, geographical location, CPC symbols, emerging focus areas, most active players (in terms of the number of patents filed / granted) . In addition, it features a patent valuation analysis which evaluates the qualitative and quantitative aspects of the patents.
- A detailed analysis of contract fill / finish services providers that offer services for prefilled syringes, featuring a list of active (large-sized) service providers and analysis based on a number of several relevant parameters, such as year of establishment, company size, scale of operation (preclinical, clinical and commercial), type of drug molecule (biologics and small molecules), and geographical location of the service provider.
- A detailed discussion on various safety features (add-on and integrated devices) installed in recent versions of prefilled syringes and the companies involved in developing and manufacturing such solutions.
- An informative summary of various guidelines established and issued by major regulatory bodies for the approval of prefilled syringes, across different countries / geographical regions.
- A brief discussion on the currently available specialty syringes, including prefilled flush syringes, prefilled diluent syringe systems and prefilled contrast agent delivery systems, along with providing details related to the various advantages offered by such devices.
- A detailed case study of companies engaged in the manufacturing of autoinjectors, featuring brief profiles of key players in this domain; each profile includes an overview of the firm, and information on its respective product portfolio.
- Elaborate profiles of prominent prefilled syringe manufacturers engaged in this domain, featuring a brief overview of the company, its financial information (if available) , information on product portfolio, recent developments and an informed future outlook.
- Elaborate profiles of prominent prefilled syringe component manufacturers, featuring a brief overview of the company, its financial information (if available) , information on product portfolio, recent developments and an informed future outlook.
One of the key objectives of the report was to evaluate the current market size and the future opportunity within the prefilled syringes market. Based on various relevant parameters, such as number of commercialized combination products, annual adoption rate, and expected pricing. We have provided an informed estimate of the likely evolution of the market for the period 2022-2035. Our year-wise projections of the current and future opportunity have further been segmented on the basis of [A] therapeutic area (blood disorders, infectious diseases, metabolic disorders, autoimmune disorders, oncological disorders, neurological disorders, ophthalmic disorders and other disorders), [B] type of syringe (specialty syringe and others), [C] type of material (glass and plastic), [D] number of chambers (single chamber and dual chamber), [E] type of drug molecule (antibodies, proteins, vaccines, small molecules, peptides, and others), and [F] key geographical regions (North America, Europe (the UK, France, Italy, Spain, Germany and the rest of Europe), Latin America (Brazil, Mexico, Argentina and the rest of Latin America), Asia Pacific (Japan, China, India, South Korea and the rest of Asia Pacific), and the Middle East and Africa (Saudi Arabia, UAE, Africa and the rest of the Middle East)).
The research, analysis and insights presented in this report are backed by a deep understanding of key insights gathered from both secondary and primary research. The opinions and insights presented in the report were influenced by discussions held with several players in this industry. The study includes detailed transcripts of discussions held with the following individuals:
- Matthew Young (Founder and Chief Technology Officer, Oval Medical Technologies)
- Kirti Maheshwari (Chief Technical Officer, Intas Pharmaceuticals)
- Gregor Kawaletz (Ex-Chief Commercial Officer, IDT Biologika)
- Tibor Hlobik (Senior Director, Product Technology Services) and Kevin Cancelliere (Ex-Directors, Pharmaceutical Delivery Systems Marketing)
- Marco Pederiva (Head of R&D and Product Strategy, Lonstroff)
- Jesse Fourt (Design Director, IDEO)
- Anonymous (Chief Executive Officer, Small-sized Medical Device Company)
All actual figures have been sourced and analyzed from publicly available information forums and primary research discussions. Financial figures mentioned in this report are in USD, unless otherwise specified.
Key Questions Answered:
- Who are the key players engaged in the development of prefilled syringes and its components?
- What are the most adopted configuration / attributes associated with the prefilled syringes?
- Which companies are actively engaged in offering fill / finish services for prefilled syringes?
- What is the relative competitiveness of different prefilled syringes that are currently available / under development?
- What are the key recent initiatives undertaken by players engaged in the manufacturing of prefilled syringes?
- How has the overall patent landscape related to prefilled syringes evolved over the past few years?
- What is the cost burden associated with the needlestick injuries and its preventions?
- What is the regulatory landscape for the approval of prefilled syringes across different geographies?
- Which companies are actively engaged in the development of combination products using prefilled syringes?
- What are the most prominent therapeutic areas being targeted by prefilled syringes?
- Who are the key opinion leaders in this domain?
- Which players are likely to partner with prefilled syringe developers in order to offer combination products?
- What are the crucial factors that are driving the prefilled syringes market?
- Which drugs are likely to be considered for administration in prefilled syringe format?
- How is the current and future market opportunity likely to be distributed across key market segments?
Chapter Outlines
Chapter 2 is an executive summary of the insights captured in our research. It offers a high-level view on the likely evolution of the prefilled syringes market in the mid to long term.
Chapter 3 provides a general overview of the prefilled syringes, including details on their origin, various affiliated components (such as barrel, lock adapter, lubricant, needle, needle shield, plunger rod / piston, plunger stopper and tip cap), fabrication material (glass and plastic), manufacturing-related information, and other critical attributes and features. Further, it highlights the opportunities associated within this domain.
Chapter 4 An overview of the current market landscape of players engaged in the manufacturing of prefilled syringes, based on several relevant parameters, such as year of establishment, company size, geographical location of the headquarters and manufacturing facilities, type of material used (glass and plastic), number of barrel chambers (single chamber and dual chamber), type of needle system (fixed needle system, luer lock and luer cone) and syringe volume.
Chapter 5 presents a detailed product competitiveness analysis of prefilled syringes, based on supplier power (in terms of employee count and annual revenues) and key product specifications (including number of chambers and needle systems, volume of the barrel and other distinguishing features).
Chapter 6 elaborates profiles of prominent prefilled syringe manufacturers engaged in this domain, featuring a brief overview of the company, its financial information (if available) , information on product portfolio, recent developments and an informed future outlook.
Chapter 7 highlights the growing concerns associated with needlestick injuries and the various steps (including regional and global legislations) that have been taken to prevent sharps related mishaps. One of the important risk mitigation strategies covered in this chapter is the installation of safety features in upcoming versions of prefilled syringes. In addition, it provides a detailed discussion on various safety features (add-on and integrated devices) installed in recent versions of prefilled syringes and the companies engaged in development and manufacturing of such solutions.
Chapter 8 provides an informative summary of various guidelines established and issued by major regulatory bodies for the approval of prefilled syringes, across different countries / geographical regions.
Chapter 9 features a detailed analysis of the key opinion leaders engaged in this domain, based on various parameters, such as (number of clinical trials, number of citations, number of publications, research gate, geographical location of the institute and research gate score).
Chapter 10 features an in-depth analysis of patents related to prefilled syringes, filed / granted, since 2015, based on several relevant parameters, such as type of patent (granted patents and patent applications), publication year, geographical location, CPC symbols, emerging focus areas, most active players (in terms of the number of patents filed / granted) . In addition, it features a patent valuation analysis which evaluates the qualitative and quantitative aspects of the patents.
Chapter 11 provides details on the various pipeline and approved products that are being evaluated or available in the form of prefilled syringes, primarily focusing on injectable drugs and vaccines. It features a detailed analysis of these drugs, based on various parameters, such as the target therapeutic area(s), phase of development, type of drug molecule, route of administration, approval year (in case of marketed products), dosage (in case of marketed products) and other approved dosage forms (in case of marketed products). In addition, the chapter provides information on drug developers engaged in this domain, along with information on their year of establishment, location of headquarters and manufacturing facilities, and strength of employee base. The chapter also includes case studies of top two marketed drugs available in prefilled syringes, namely Humira® and Enbrel®.
Chapter 12 features a discussion on the most commonly targeted therapeutic indications, including information on the approved / marketed injectable drug products available for the treatment of such clinical conditions (across different target therapeutic areas) and their respective biosimilars.
Chapter 13 presents an in-depth analysis of marketed drugs / therapies and pipeline candidates that are likely to be available in prefilled syringe format in the near future, based on several relevant parameters, such as route of administration, type of drug molecule, target indication, other available dosage forms (for approved drugs) and historical annual sales information (for approved drugs). Additionally, the chapter features an insightful analysis, highlighting potential strategic partners (primarily drug developers) for prefilled syringes manufacturers, based on multiple parameters, such as pipeline strength, targeted therapeutic indications, and developer strength. The analysis aims to provide the necessary inputs to the latter type of stakeholders, enabling them to make the right decisions to develop combination products.
Chapter 14 features a review of the various prefilled syringe combination product-related initiatives undertaken by big pharma players (shortlisted on the basis of revenue generated in 2020), based on various parameters, such as current adoption (based on number of approved and under development prefilled syringe combination products) and target therapeutic area(s) and type of drug molecule.
Chapter 15 features information on specialty syringes, namely prefilled flush syringes, prefilled diluent systems and prefilled contrast agent delivery systems. For each type of specialty syringe, we have provided a brief overview and details on the different products that are available in the market and their advantages.
Chapter 16 discusses the recent technological developments in this domain and their applications. The chapter provides details on the advancements in design technology and manufacturing of prefilled syringes that have enabled pharmaceutical companies to market lyophilized drugs in dual chambered syringe systems. The chapter also highlights novel lubrication and sterilization technologies.
Chapter 17 presents an insightful market forecast analysis, highlighting the future potential of the prefilled syringes market till the year 2035. Our year-wise projections of the current and future opportunity have further been segmented on the basis of [A] therapeutic area (blood disorders, infectious diseases, metabolic disorders, autoimmune disorders, oncological disorders, neurological disorders, ophthalmic disorders and other disorders), [B] type of syringe (specialty syringes and other syringes), [C] type of material (glass and plastic), [D] number of chambers (single chamber and dual chamber), [E] type of drug molecule (antibodies, proteins, vaccines, small molecules, peptides, and others), and [F] key geographical regions (North America, Europe (the UK, France, Italy, Spain, Germany and the rest of Europe), Latin America (Brazil, Mexico, Argentina and the rest of Latin America), Asia Pacific (Japan, China, India, South Korea and the rest of Asia Pacific), and the Middle East and Africa (Saudi Arabia, UAE, Africa and the rest of the Middle East)).
Chapter 18 features a detailed discussion on the various factors that are anticipated to drive growth within the prefilled syringes market, such as the rise in preference for self-administration of medication, increase in incidence of chronic diseases, evolving patient landscape, introduction and growing adoption of biologics / biosimilars, initiatives focused on the prevention of needlestick injuries and the various potential applications of prefilled syringes.
Chapter 19 provides a detailed SWOT analysis of the prefilled syringes market. The chapter presents strategic insights on major factors that have contributed to the growth of the market, while highlighting the weaknesses and threats that are likely to have an impact on its future.
Chapter 20 Elaborate profiles of prominent prefilled syringe component manufacturers, featuring a brief overview of the company, its financial information (if available) , information on product portfolio, recent developments and an informed future outlook.
Chapter 21 A detailed analysis of contract fill / finish services providers that offer services for prefilled syringes, featuring a list of active (large-sized) service providers and analysis based on a number of several relevant parameters, such as year of establishment, company size, scale of operation (preclinical, clinical and commercial), type of drug molecule (biologics and small molecules), and geographical location of the service provider.
Chapter 22 A detailed case study of companies engaged in the manufacturing of autoinjectors, featuring brief profiles of key players in this domain; each profile includes an overview of the firm, and information on its respective product portfolio.
Chapter 23 is a summary of the overall report. In this chapter, we have provided a list of key takeaways from the report, and expressed our independent opinion related to the research and analysis described in the previous chapters.
Chapter 24 is a collection of transcripts of interviews conducted with representatives from renowned organizations that are engaged in the prefilled syringes manufacturing domain. In this chapter, we have presented the details of our conversations with Matthew Young (Founder and Chief Technology Officer, Oval Medical Technologies), Kirti Maheshwari (Chief Technical Officer, Intas Pharmaceuticals), Gregor Kawaletz (Ex-Chief Commercial Officer, IDT Biologika), Kevin Cancelliere (Senior Directors, Product Technology Services) and Tibor Hlobik (Ex-Directors, Pharmaceutical Delivery Marketing System), Marco Pederiva (Marketing & Sales Director, Lonstroff) and Anonymous (Chief Executive Officer, Small-sized Medical Device Company).
Chapters 25 is an appendix that provides tabulated data and numbers for all the figures included in the report.
Chapter 26 is an appendix that contains the list of companies and organizations mentioned in the report.
TABLE OF CONTENTS
1. PREFACE
- 1.1. Chapter Overview
- 1.2. Scope of the Report
- 1.3. Research Methodology
- 1.4 Key Questions Answered
- 1.5. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Historical Evolution of Prefilled Syringes
- 3.3. Benefits of Prefilled Syringes Over Traditional Injectable Devices
- 3.3.1. Benefits of Prefilled Syringes for Healthcare Professionals and End Users
- 3.3.2. Benefits of Prefilled Syringes for Manufacturers
- 3.3.3. Transition from Existing Dosage Forms to Prefilled Syringes
- 3.4. Prefilled Syringe Components
- 3.5. Classification of Prefilled Syringes
- 3.5.1. Based on Barrel Fabrication Material
- 3.5.1.1. Glass Barrel Prefilled Syringes
- 3.5.1.1.1. Limitations of Glass Barrel Prefilled Syringes
- 3.5.1.1.2. Addressing the Limitations of Glass Barrel Prefilled Syringes
- 3.5.1.2. Plastic
- 3.5.1.2.1. Limitations of Plastic Barrel Prefilled Syringes
- 3.5.1.2.2. Addressing the Limitations of Plastic Barrel Prefilled Syringes
- 3.5.1.2.3. Factors Likely to Drive the Use of Plastic Barrel Prefilled Syringes
- 3.5.1.1. Glass Barrel Prefilled Syringes
- 3.5.2. Based on Number of Chambers in the Barrel
- 3.5.3. Based on Type of Needle System
- 3.5.4. Based on Type of Packaging
- 3.5.1. Based on Barrel Fabrication Material
- 3.6. Critical Attributes of Prefilled Syringe Design
- 3.7. Key Manufacturing Steps for Prefilled Syringes
- 3.7.1. Production of Barrels
- 3.7.1.1. Glass Barrel Prefilled Syringes
- 3.7.1.2. Plastic Barrel Prefilled Syringes
- 3.7.2. Production of Syringes
- 3.7.3. Barrel Siliconization
- 3.7.4. Syringe Sterilization
- 3.7.5. Validation of Sterilization
- 3.7.6. Syringe Filling
- 3.7.7. Syringe Testing
- 3.7.1. Production of Barrels
- 3.8. Future Perspectives
4. PREFILLED SYRINGES: MARKET OVERVIEW
- 4.1. Chapter Overview
- 4.2. Prefilled Syringes: List of Available / Under Development Devices
- 4.2.1. Analysis by Type of Barrel Fabrication Material
- 4.2.2. Analysis by Number of Barrel Chambers
- 4.2.3. Analysis by Type of Needle System
- 4.2.4. Analysis by Device Capacity
- 4.3. Prefilled Syringes: List of Developers
- 4.3.1. Analysis by Year of Establishment
- 4.3.2. Analysis by Company Size
- 4.3.3. Analysis by Location of Headquarters
- 4.3.4. Analysis by Company Size and Location of Headquarters
- 4.3.5. Analysis by Location of Manufacturing Facilities
- 4.3.6. Most Active Players: Analysis by Number of Products
- 4.4. Technological Developments Related to Prefilled Syringes
5. PRODUCT COMPETITIVENESS ANALYSIS
- 5.1. Chapter Overview
- 5.2. Assumptions and Key Parameters
- 5.3. Methodology
- 5.4. Product Competitiveness Analysis: Prefilled Syringes
- 5.4.1. Glass Barrel Prefilled Syringes
- 5.4.2. Plastic Barrel Prefilled Syringes
6. PREFILLED SYRINGE MANUFACTURERS
- 6.1. Chapter Overview
- 6.2. Prefilled Syringe Manufacturers Based in North America
- 6.2.1. Becton Dickinson
- 6.2.1.1. Company Overview
- 6.2.1.2. Financial Performance
- 6.2.1.3. Prefilled Syringe Portfolio
- 6.2.1.4. Glass Barrel Prefilled Systems
- 6.2.1.4.1. BD Hypak™ Prefilled Syringe
- 6.2.1.4.2. BD Neopak™ Prefilled Syringe
- 6.2.1.4.3. BD Hylok™ Prefilled Syringe
- 6.2.1.5. Plastic Barrel Prefilled Systems
- 6.2.1.5.1. BD Sterifill Syringe Systems
- 6.2.1.5.2 BD PosiFlush® Syringe Systems
- 6.2.1.6. Other Prefilled Delivery Systems
- 6.2.1.6.1. BD Uniject®
- 6.2.1.7. Future Outlook
- 6.2.2 Pfizer Hospital
- 6.2.2.1. Company Overview
- 6.2.2.2. Financial Performance
- 6.2.2.3 Prefilled Syringe Portfolio
- 6.2.2.3.1. ABBOJECT® Syringe
- 6.2.2.3.2. Emergency Syringe
- 6.2.2.3.3. iSecure™ Syringe System
- 6.2.2.3.4. NEXJECT™ Syringe
- 6.2.2.4. Future Outlook
- 6.2.3. West Pharmaceutical
- 6.2.3.1. Company Overview
- 6.2.3.2. Financial Performance
- 6.2.3.3. Prefilled Technology Solutions
- 6.2.3.4. Plastic Prefilled Systems
- 6.2.3.4.1. Daikyo Crystal Zenith® Polymer RTU Prefilled Syringe
- 6.2.3.5. Drug Reconstitution Systems
- 6.2.3.6 History of CZ Syringe
- 6.2.3.7. Manufacturing Focused Expansions
- 6.2.3.8. Future Outlook
- 6.2.1. Becton Dickinson
- 6.3. Key Prefilled Syringe Manufacturers Based in Europe
- 6.3.1. Gerresheimer
- 6.3.1.1. Company Overview
- 6.3.1.2. Financial Performance
- 6.3.1.3. Prefilled Technology Solutions
- 6.3.1.4. Glass Prefilled Systems
- 6.3.1.4.1. Gx Glass Syringes
- 6.3.1.5. Plastic Prefilled Systems
- 6.3.1.5.1. ClearJect® Prefilled syringes
- 6.3.1.5.2. Gx RTF® Clearject® Needle Syringe
- 6.3.1.6. Safety Syringes
- 6.3.1.6.1. Gx InnoSafe® Safety Syringes
- 6.3.1.7. Self-Injection Devices
- 6.3.1.8. Key Developments
- 6.3.1.9. Future Outlook
- 6.3.2. Ompi
- 6.3.2.1. Company Overview
- 6.3.2.2. Financial Performance
- 6.3.2.3. Glass Barrel Prefilled Systems
- 6.3.2.3.1. EZ-fill® Syringes
- 6.3.2.3.1.1. EZ-fill® Fina
- 6.3.2.3.1.2. EZ-fill® Nexa
- 6.3.2.3.1.3. EZ-fill® Alba
- 6.3.2.3.1. EZ-fill® Syringes
- 6.3.2.4. Key Developments
- 6.3.2.5. Future Outlook
- 6.3.3. Schott
- 6.3.3.1. Company Overview
- 6.3.3.2. Financial Performance
- 6.3.3.3. Glass Barrel Prefilled Syringes
- 6.3.3.3.1. syriQ® Glass Syringes
- 6.3.3.4. Plastic Barrel Prefilled Syringes
- 6.3.3.4.1. TopPac® Polymer Syringes
- 6.3.3.5. Product Portfolio
- 6.3.3.5.1. SyriQ® BioPure Syrigne System for Sensitive Drugs
- 6.3.3.5.2. TopPac® Syringe System for Sensitive Drugs
- 6.3.3.5.3. TopPac® Syringe System for Highly Viscous Drugs
- 6.3.3.5.4. TopPac® 20mL Syringe System for Infusion Therapy
- 6.3.3.6. Key Developments
- 6.3.3.7. Future Outlook
- 6.3.1. Gerresheimer
- 6.4. Key Prefilled Syringe Manufacturers in Asia
- 6.4.1. Nipro (Acquired MGlas)
- 6.4.1.1. Company Overview
- 6.4.1.2. Financial Performance
- 6.4.1.3. Glass Barrel Prefilled Systems
- 6.4.1.4. Key Developments
- 6.4.1.5. Future Outlook
- 6.4.2. Taisei Kako
- 6.4.2.1. Company Overview
- 6.4.2.2. Glass Barrel Prefilled Systems
- 6.4.2.2.1. VF-Syringe
- 6.4.2.2.2. SIN-Syringe
- 6.4.2.3. Plastic Barrel Prefilled System
- 6.4.2.3.1. ClearJect® Syringes
- 6.4.2.3.2. FULJECT Passive Type Syringe
- 6.4.2.4. Future Outlook
- 6.4.1. Nipro (Acquired MGlas)
- 6.5. Other Manufacturers
- 6.5.1. Aguettant
- 6.5.1.1. Company Overview
- 6.5.1.2. Prefilled Syringe Portfolio
- 6.5.1.2.1. Aguettant Prefilled Syringe
- 6.5.1.3. Key Developments
- 6.5.1.4. Future Outlook
- 6.5.2. Arte
- 6.5.2.1. Company Overview
- 6.5.2.2. Prefilled Syringe Portfolio
- 6.5.2.2.1. ARTE Prefillable Syringe
- 6.5.2.2.2. Dual Chamber Prefillable Syringe
- 6.5.2.2.3. Single Chamber Prefillable Syringe
- 6.5.2.3. Future Outlook
- 6.5.3. J.O. Pharma (Subsidiary of Otsuka Holdings)
- 6.5.3.1. Company Overview
- 6.5.3.2. Prefilled Syringe Portfolio
- 6.5.3.3. Key Developments
- 6.5.3.4 Future Outlook
- 6.5.4. Shandong Pharmaceutical Glass
- 6.5.4.1. Company Overview
- 6.5.4.2. Prefilled Syringes Portfolio
- 6.5.4.3 Future Outlook
- 6.5.5. Shandong Zibo Minkang Pharmaceutical packing
- 6.5.5.1. Company Overview
- 6.5.5.2. Prefilled Syringes Portfolio
- 6.5.5.3. Future Outlook
- 6.5.6. Terumo
- 6.5.6.1. Company Overview
- 6.5.6.2. Financial Performance
- 6.5.6.3. PLAJEX™ (Plastic Prefilled Syringe)
- 6.5.6.4. Safety Add-On Devices
- 6.5.6.5. Key Developments
- 6.5.6.6. Future Outlook
- 6.5.7. Vetter Pharma
- 6.5.7.1. Company Overview
- 6.5.7.2. Prefilled Syringe Portfolio
- 6.5.7.2.1. Lyo-Ject® Glass Prefilled Syringe
- 6.5.7.2.2. Vetter-Ject® Safety Device
- 6.5.7.2.3. V-OVS Safety Device
- 6.5.7.3. Key Developments
- 6.5.7.4. Future Outlook
- 6.5.8. WEGO Prefills Pharmaceutical Packaging
- 6.5.8.1. Company Overview
- 6.5.8.2. Financial Performance
- 6.5.8.3. WeGo Glass Prefilled Syringe
- 6.5.8.4. Weigao Prefilled Flush Syringes
- 6.5.8.5. Future Outlook
- 6.5.1. Aguettant
7. NEEDLESTICK INJURIES
- 7.1. Chapter Overview
- 7.2. Incidence and Associated Financial Burden
- 7.3. Government Legislations for Prevention of Needlestick Injuries
- 7.4. Safety Mechanisms Used in Modern Prefilled Syringes
- 7.4.1. Safety Systems: Add-On Safety Device Manufacturers
- 7.4.1.1. Becton Dickinson
- 7.4.1.2. Catalent
- 7.4.1.3. Terumo
- 7.4.1.4. Tip-Top
- 7.4.1.5. West Pharmaceutical
- 7.4.2. Safety Systems: Integrated Safety Device Manufacturers
- 7.4.2.1. Gerresheimer
- 7.4.2.2. Injecto
- 7.4.2.3. MedicalChain International
- 7.4.2.4. OMPI
- 7.4.2.5. Owen Mumford
- 7.4.2.6. SHL Group
- 7.4.2.7. Taisei Kako
- 7.4.1. Safety Systems: Add-On Safety Device Manufacturers
8. REGULATORY LANDSCAPE FOR PREFILLED SYRINGES
- 8.1. Chapter Overview
- 8.2. Regulatory Approval of Combination Products in the US
- 8.2.1. Overview
- 8.2.2. Historical Background
- 8.2.3. Role of Regulatory Bodies in Product Approval
- 8.2.4. Regulatory Approval for Prefilled Syringes
- 8.2.4.1. Reimbursement Landscape
- 8.2.4.2. Payer Mix
- 8.2.4.3. Reimbursement Process
- 8.3. Regulatory Approval of Combination Products in Europe
- 8.3.1. Overview
- 8.3.2. Role of Regulatory Bodies in Product Approval
- 8.3.3. Regulatory Approval for Prefilled Syringes
- 8.3.3.1. Reimbursement Landscape for the UK
- 8.3.3.2. Payer Mix
- 8.3.3.3. Reimbursement Process in the UK
- 8.3.3.4. Reimbursement Landscape for France
- 8.3.3.5. Payer Mix
- 8.3.3.6. Reimbursement Process in France
- 8.3.3.7. Reimbursement Landscape for Spain
- 8.3.3.8. Payer Mix
- 8.3.3.9. Reimbursement Process in Spain
- 8.3.3.10. Reimbursement Landscape for Germany
- 8.3.3.11. Payer Mix
- 8.3.3.12. Reimbursement Process in Germany
- 8.3.3.13. Reimbursement Landscape for Italy
- 8.3.3.14. Payer Mix
- 8.3.3.15. Reimbursement Process in Italy
- 8.4. Regulatory Approval of Combination Products in Canada
- 8.4.1. Overview
- 8.4.2. Role of Regulatory Bodies in Product Approval
- 8.4.3. Regulatory Approval for Prefilled Syringes
- 8.4.3.1. Reimbursement Landscape for Canada
- 8.4.3.2. Payer Mix
- 8.4.3.3. Reimbursement Process in Canada
- 8.5. Regulatory Approval of Combination Products in Brazil
- 8.5.1. Overview
- 8.5.2. Role of Regulatory Bodies in Product Approval
- 8.5.3. Regulatory Approval for Prefilled Syringes
- 8.5.3.1. Reimbursement Landscape for Brazil
- 8.5.3.2. Payer Mix
- 8.5.3.3. Reimbursement Process in Brazil
- 8.6. Regulatory Approval of Combination Products in Australia
- 8.6.1. Overview
- 8.6.2. Role of Regulatory Bodies in Product Approval
- 8.6.3. Regulatory Approval for Prefilled Syringes
- 8.6.3.1. Reimbursement Landscape for Australia
- 8.6.3.2. Payer Mix
- 8.6.3.3. Reimbursement Process in Australia
- 8.7. Regulatory Approval of Combination Products in Japan
- 8.7.1. Overview
- 8.7.2. Role of Regulatory Bodies in Product Approval
- 8.7.3. Regulatory Approval for Prefilled Syringes
- 8.7.3.1. Reimbursement Landscape for Japan
- 8.7.3.2. Payer Mix
- 8.7.3.3. Reimbursement Process in Japan
- 8.8. Regulatory Approval of Combination Products in China
- 8.8.1. Overview
- 8.8.2. Role of Regulatory Bodies in Product Approval
- 8.8.3. Regulatory Approval for Prefilled syringes
- 8.8.3.1. Reimbursement Landscape for China
- 8.8.3.2. Payer Mix
- 8.8.3.3. Reimbursement Process in China
- 8.9. Regulatory Approval of Combination Products in India
- 8.9.1. Overview
- 8.9.2. Role of Regulatory Bodies in Product Approval
- 8.9.3. Regulatory Approval for Prefilled Syringes
- 8.9.3.1. Reimbursement Landscape for India
- 8.9.3.2. Payer Mix
- 8.10. Regulatory Approval of Combination Products in South Korea
- 8.10.1. Overview
- 8.10.2. Role of Regulatory Bodies in Product Approval
- 8.10.3. Regulatory Approval for Prefilled Syringes
- 8.10.3.1. Reimbursement Landscape for Saudi Arabia
- 8.10.3.2. Payer Mix
- 8.10.3.3. Reimbursement Process in Saudi Arabia
- 8.11. Regulatory Approval of Combination Products in United Arab Emirates
- 8.11.1. Overview
- 8.11.2. Role of Regulatory Bodies in Product Approval
- 8.11.3. Regulatory Approval for Prefilled Syringes
- 8.11.3.1. Reimbursement Landscape for United Arab Emirates
- 8.11.3.2. Payer Mix
- 8.11.3.3. Reimbursement Process in United Arab Emirates
9. PREFILLED SYRINGE COMBINATION PRODUCTS: MARKET OVERVIEW
- 9.1. Chapter Overview
- 9.2. Prefilled Syringe Combination Products: List of Approved Drugs
- 9.2.1. Analysis by Type of Drug Molecule
- 9.2.2. Analysis by Approval Year
- 9.2.3. Analysis by Geography
- 9.2.4. Analysis by Route of Administration
- 9.2.5. Analysis by Target Therapeutic Area
- 9.2.6. Prefilled Syringe Combination Products: Additional Information
- 9.2.6.1. Analysis by Dose Strength
- 9.2.6.2. Analysis by Other Approved Dosage Forms
- 9.3. Prefilled Syringe Combination Products: List of Clinical Stage Drugs
- 9.3.1. Analysis by Type of Drug Molecule
- 9.3.2. Analysis by Phase of Development
- 9.3.3. Analysis by Route of Administration
- 9.3.4. Analysis by Target Therapeutic Area
- 9.4. Prefilled Syringe Combination Products: List of Developers
- 9.4.1. Analysis by Year of Establishment
- 9.4.2. Analysis by Company Size
- 9.4.3. Analysis by Location of Headquarters
- 9.5. Leading Drugs Available in Prefilled Syringe Format
- 9.6. Other Drugs Available in Prefilled Syringe Format
- 9.7. Case Study of Popular Drugs Available in Prefilled Syringe Market
- 9.7.1. HUMIRA® (Adalimumab), AbbVie / Eisai
- 9.7.1.1. Target Indications and Available Dosage Forms
- 9.7.1.2. Shift from Vials to Syringes
- 9.7.2. Enbrel® (Etanercept), Amgen / Pfizer / Takeda
- 9.7.2.1. Target Indications and Available Dosage Forms
- 9.7.2.2. Shift from Vials to Syringes
- 9.7.1. HUMIRA® (Adalimumab), AbbVie / Eisai
10. KEY THERAPEUTIC AREAS
- 10.1. Chapter Overview
- 10.1.1. Autoimmune Disorders
- 10.1.1.1. Approved Injectables
- 10.1.1.2. Biosimilars
- 10.1.2. Infectious Diseases
- 10.1.2.1. Antiviral Drugs
- 10.1.2.1.1. Approved Injectables
- 10.1.2.1.2. Biosimilars
- 10.1.2.2. Vaccines
- 10.1.2.2.1. Approved Injectables
- 10.1.2.1. Antiviral Drugs
- 10.1.3. Neurological Disorders
- 10.1.3.1. Approved Injectables
- 10.1.3.2. Biosimilars
- 10.1.4. Metabolic Disorders
- 10.1.4.1. Approved Injectables
- 10.1.4.2. Biosimilars
- 10.1.1. Autoimmune Disorders
11. PREFILLED SYRINGES: LIKELY DRUG CANDIDATES AND PARTNER ANALYSIS
- 11.1. Chapter Overview
- 11.2. Likely Drug Candidates
- 11.2.1. Methodology and Key Parameters
- 11.3. Marketed Drug Candidates
- 11.3.1. Most Likely Candidates for Delivery via Prefilled Syringes
- 11.3.2. Likely Candidates for Delivery via Prefilled Syringes
- 11.3.3. Less Likely Candidates for Delivery via Prefilled Syringes
- 11.3.4. Least Likely Candidates for Delivery via Prefilled Syringes
- 11.4. Clinical Drug Candidates
- 11.4.1. Most Likely Candidates for Delivery via Prefilled Syringes
- 11.4.2. Likely Candidates for Delivery via Prefilled Syringes
- 11.4.3. Less Likely Candidates for Delivery via Prefilled Syringes
- 11.4.4. Least Likely Candidates for Delivery via Prefilled Syringes
- 11.5. Potential Strategic Partners
- 11.5.1. Methodology and Key Parameters
- 11.5.2. Potential Strategic Partners in North America
- 11.5.2.1. Most Likely Partners for Prefilled Syringes Combination Products
- 11.5.2. 2. Likely Partners for Prefilled Syringes Combination Products
- 11.5.2.3. Less Likely Partners for Prefilled Syringes Combination Products
- 11.5.2.4. Least Likely Partners for Prefilled Syringes Combination Products
- 11.5.3. Potential Strategic Partners in Europe
- 11.5.3.1. Most Likely Partners for Prefilled Syringes Combination Products
- 11.5.3.2. Likely Partners for Prefilled Syringes Combination Products
- 11.5.3.3. Less Likely Partners for Prefilled Syringes Combination Products
- 11.5.3.4. Least Likely Partners for Prefilled Syringes Combination Products
- 11.5.4. Potential Strategic Partners in Asia-Pacific and Rest of the World
- 11.5.4.1. Most Likely Partners for Prefilled Syringes Combination Products
- 11.5.4.2. Likely Partners for Prefilled Syringes Combination Products
- 11.5.4.3. Less Likely Partners for Prefilled Syringes Combination Products
- 11.5.4.4. Least Likely Partners for Prefilled Syringes Combination Products
12. BIG PHARMA INITIATIVES: PREFILLED SYRINGE COMBINATION PRODUCTS
- 12.1. Chapter Overview
- 12.2. Methodology
- 12.3. Key Pharmaceutical Companies
- 12.3.1. Analysis by Therapeutic Area
- 12.3.1.1. Autoimmune Disorders
- 12.3.1.2. Infectious Diseases
- 12.3.1.3. Oncological Disorders
- 12.3.1.4. Metabolic Disorders
- 12.3.1.5. Neurological Disorders
- 12.3.1.6. Inflammatory Disorders
- 12.3.1.7. Orthopedic Disorders
- 12.3.1.8. Cardiovascular Disorders
- 12.3.1.9. Respiratory Disorders
- 12.3.1.10. Ophthalmic Disorders
- 12.3.1.11. Others
- 12.3.2. Analysis by Type of Molecule
- 12.3.2.1. Antibodies
- 12.3.2.2. Vaccines
- 12.3.2.3. Proteins
- 12.3.2.4. Small Molecules
- 12.3.2.5. Peptides
- 12.3.2.4. Others
- 12.3.1. Analysis by Therapeutic Area
13. RECENT DEVELOPMENTS
- 13.1. Chapter Overview
- 13.2. List of Recent Initiatives Related to Prefilled Syringes, 2019-2021
- 13.3. Analysis by Year of Initiative
- 13.4. Analysis by Type of Initiative
- 13.5. Analysis by Year and Type of Initiative
- 13.5.1 Analysis by Type of Expansion
- 13.5.2 Analysis by Type of Investment and Funding
- 13.5.3 Analysis by Type of Partnership
- 13.6. Analysis by Geography
- 13.7. Most Active Players: Distribution by Number of Initiatives
- 13.8. Analysis of Player by Type of Instances
14. PATENT ANALYSIS
- 14.1 Chapter Overview
- 14.2. Patent Analysis: Prefilled Syinges
- 14.2.1. Scope and Methodology
- 14.2.2. Analysis by Publication Year
- 14.2.3. Analysis by Publication Year and Type of Patent
- 14.2.4. Analysis by Geography
- 14.2.5. Analysis by CPC Symbols
- 14.2.6. Analysis by Emerging Focus Area
- 14.2.7. Most Active Players: Analysis by Number of Patents
- 14.2.8. Patent Benchmarking Analysis
- 14.2.9. Overall Intellectual Property Portfolio: Analysis by Type of Organization
- 14.2.10. Patent Valuation Analysis
15. SPECIALTY PREFILLED SYRINGES
- 15.1. Chapter Overview
- 15.2. Prefilled Flush Syringes
- 15.2.1. Overview
- 15.2.2. Prefilled Flush Syringes Available in the Market
- 15.2.3. Advantages of Prefilled Flush Syringes
- 15.3. Prefilled Diluent Syringes
- 15.3.1. Overview
- 15.3.2. Lyophilized Drugs Available in Prefilled Diluent Syringes
- 15.3.3. Advantages of Prefilled Diluent Syringes
- 15.4. Contrast Agent Prefilled Syringes
- 15.4.1. Overview
- 15.4.2. Contrast Agents Available in Prefilled Syringes
- 15.4.3. Advantages of Contrast Agents Used in Prefilled Syringes
16. TECHNOLOGICAL ADVANCES RELATED TO PREFILLED SYRINGE
- 16.1. Chapter Overview
- 16.2. Prefilled Syringes for Lyophilized Drugs
- 16.2.1. Prefilled Diluent Syringes
- 16.2.2. Prefilled Dual / Multi-Chamber Prefilled Syringes
- 16.2.2.1. Lyophilized Drugs Available in Dual / Multi-Chamber Prefilled Syringes
- 16.2.2.2. Prefilled Dual-Chambered Pen Injectors for Lyophilized Drugs
- 16.3. Prefilled Syringes for Ophthalmic Administration
- 16.4. Prefilled Syringes for Dermal Fillers
- 16.5. Multilayer Plastic Prefilled Syringes with Oxygen Barrier
- 16.6. Prefilled Syringes with Low Particle Formation Risk
- 16.7. Lubrication Technology for Prefilled Syringes
- 16.8. Advances in Terminal Sterilization of Prefilled Syringes
- 16.8.1. Noxilizer's Nitrogen Dioxide Sterilization Technology
- 16.9. Prefilled Syringe Usage Aids for Patients and Healthcare Providers
17. MARKET SIZING AND OPPORTUNITY ANALYSIS
- 17.1. Chapter Overview
- 17.2. Scope and Methodology
- 17.3. Global Prefilled Syringes Market, 2022-2035
- 17.3.1. Prefilled Syringes Market: Analysis by Therapeutic Area
- 17.3.1.1. Prefilled Syringes Market for Autoimmune Disorders, 2022-2035
- 17.3.1.2. Prefilled Syringes Market for Infectious Diseases, 2022-2035
- 17.3.1.3. Prefilled Syringes Market for Neurological Disorders, 2022-2035
- 17.3.1.4. Prefilled Syringes Market for Blood Disorders, 2022-2035
- 17.3.1.5. Prefilled Syringes Market for Oncological Disorders, 2022-2035
- 17.3.1.6. Prefilled Syringes Market for Psychiatric Diseases, 2022-2035
- 17.3.1.7. Prefilled Syringes Market for Respiratory Disorders, 2022-2035
- 17.3.1.8. Prefilled Syringes Market for Cardiovascular Disorders, 2022-2035
- 17.3.1.9. Prefilled Syringes Market for Metabolic Disorders, 2022-2035
- 17.3.1.10. Prefilled Syringes Market for Ophthalmic Disorder, 2022-2035
- 17.3.1.11. Prefilled Syringes Market for Orthopedic Disorders, 2022-2035
- 17.3.1.12. Prefilled Syringes Market for Other Diseases, 2022-2035
- 17.3.2. Prefilled Syringes Market: Analysis by Type of Syringe Barrel Material
- 17.3.2.1. Prefilled Syringes Market for Glass Barrel Prefilled Syringes, 2022-2035
- 17.3.2.2. Prefilled Syringes Market for Plastic Barrel Prefilled Syringes, 2022-2035
- 17.3.3. Prefilled Syringes Market: Analysis by Type of Chamber System
- 17.3.3.1. Prefilled Syringes Market for Single Chamber Prefilled Syringes, 2022-2035
- 17.3.3.2. Prefilled Syringes Market for Dual Chamber Prefilled Syringes, 2022-2035
- 17.3.4. Prefilled Syringes Market: Analysis by Type of Drug Molecule
- 17.3.4.1. Prefilled Syringes Market for Antibodies, 2022-2035
- 17.3.4.2. Prefilled Syringes Market for Proteins, 2022-2035
- 17.3.4.3. Prefilled Syringes Market for Peptides, 2022-2035
- 17.3.4.4. Prefilled Syringes Market for Small Molecules, 2022-2035
- 17.3.4.5. Prefilled Syringes Market for Vaccines, 2022-2035
- 17.3.5. Prefilled Syringes Market: Analysis by Geography
- 17.3.5.1. Prefilled Syringes Market for North America, 2022-2035
- 17.3.5.2. Prefilled Syringes Market for Europe, 2022-2035
- 17.3.5.3. Prefilled Syringes Market for Asia Pacific, 2022-2035
- 17.3.5.4. Prefilled Syringes Market for Latin America, 2022-2035
- 17.3.5.5. Prefilled Syringes Market for Middle East and Africa, 2022-2035
- 17.3.6. Prefilled Syringes Market: Analysis by Specialty Syringes
- 17.3.7. Prefilled Syringes Market for Autoimmune Disorders, 2022-2035
- 17.3.7.1. Prefilled Syringes Market for Autoimmune Disorders: Analysis by Syringe Barrel Material
- 17.3.7.1.1. Glass Barrel Prefilled Syringes Market for Autoimmune Disorders
- 17.3.7.1.2. Plastic Barrel Prefilled Syringes Market for Autoimmune Disorders
- 17.3.7.2. Prefilled Syringes Market for Autoimmune Disorders: Analysis by Type of Chamber System
- 17.3.7.2.1. Single Chamber Prefilled Syringes Market for Autoimmune Disorders
- 17.3.7.2.2. Dual Chamber Prefilled Syringes Market for Autoimmune Disorders
- 17.3.7.3. Prefilled Syringes Market for Autoimmune Disorders: Analysis by Type of Drug Molecule
- 17.3.7.3.1. Antibodies Market for Autoimmune Disorders
- 17.3.7.3.2. Proteins Market for Autoimmune Disorders
- 17.3.7.3.3. Small Molecules Market for Autoimmune Disorders
- 17.3.7.4. Prefilled Syringes Market for Autoimmune Disorders: Analysis by Geography
- 17.3.7.4.1. North America Market for Autoimmune Disorders
- 17.3.7.4.2. Europe Market for Autoimmune Disorders
- 17.3.7.4.3. Asia Pacific Market for Autoimmune Disorders
- 17.3.7.4.4. Latin America Market for Autoimmune Disorders
- 17.3.7.4.5. Middle East and Africa Market for Autoimmune Disorders
- 17.3.7.1. Prefilled Syringes Market for Autoimmune Disorders: Analysis by Syringe Barrel Material
- 17.3.8. Prefilled Syringes Market for Infectious Disorders, 2022-2035
- 17.3.8.1. Prefilled Syringes Market for Infectious Disorders: Analysis by Type of Syringe Barrel Material
- 17.3.8.1.1. Glass Barrel Prefilled Syringes Market for Infectious Disorders
- 17.3.8.1.2. Plastic Barrel Prefilled Syringes Market for Infectious Disorders
- 17.3.8.2. Prefilled Syringes Market for Infectious Disorders: Analysis by Type of Chamber System
- 17.3.8.2.1. Single Chamber Prefilled Syringes Market for Infectious Disorders
- 17.3.8.2.2. Dual Chamber Prefilled Syringes Market for Infectious Disorders
- 17.3.8.3. Prefilled Syringes Market for Infectious Disorders: Analysis by Type of Drug Molecule
- 17.3.8.3.1. Vaccines Market for Infectious Disorders
- 17.3.8.4. Prefilled Syringes Market for Infectious Disorders: Analysis by Geography
- 17.3.8.4.1. North America Market for Infectious Disorders
- 17.3.8.4.2. Europe Market for Infectious Disorders
- 17.3.8.4.3. Asia Pacific Market for Infectious Disorders
- 17.3.8.4.4. Latin America Market for Infectious Disorders
- 17.3.8.4.5. Middle East and Africa Market for Infectious Disorders
- 17.3.8.1. Prefilled Syringes Market for Infectious Disorders: Analysis by Type of Syringe Barrel Material
- 17.3.9. Prefilled Syringes Market for Neurological Disorders, 2022-2035
- 17.3.9.1. Prefilled Syringes Market for Neurological Disorders: Analysis by Type of Syringe Barrel Material
- 17.3.9.1.1. Glass Barrel Prefilled Syringes Market for Neurological Disorders
- 17.3.9.1.2. Plastic Barrel Prefilled Syringes Market for Neurological Disorders
- 17.3.9.2. Prefilled Syringes Market for Neurological Disorders: Analysis by Type of Chamber System
- 17.3.9.2.1. Single Chamber Prefilled Syringes Market for Neurological Disorders
- 17.3.9.2.2. Dual Chamber Prefilled Syringes Market for Neurological Disorders
- 17.3.9.3. Prefilled Syringes Market for Neurological Disorders: Analysis by Type of Drug Molecule
- 17.3.9.3.1. Antibodies Market for Neurological Disorders
- 17.3.9.3.2. Proteins Market for Neurological Disorders
- 17.3.9.3.3. Peptides Market for Neurological Disorders
- 17.3.9.4. Prefilled Syringes Market for Neurological Disorders: Analysis by Geography
- 17.3.9.4.1. North America Market for Neurological Disorders
- 17.3.9.4.2. Europe Market for Neurological Disorders
- 17.3.9.4.3. Asia Pacific Market for Neurological Disorders
- 17.3.9.4.4. Latin America Market for Neurological Disorders
- 17.3.9.4.5. Middle East and Africa Market for Neurological Disorders
- 17.3.9.1. Prefilled Syringes Market for Neurological Disorders: Analysis by Type of Syringe Barrel Material
- 17.3.10. Prefilled Syringes Market for Blood Disorders, 2022-2035
- 17.3.10.1. Prefilled Syringes Market for Blood Disorders: Analysis by Type of Syringe Barrel Material
- 17.3.10.1.1. Glass Barrel Prefilled Syringes Market for Blood Disorders
- 17.3.10.1.2. Plastic Barrel Prefilled Syringes Market for Blood Disorders
- 17.3.10.2. Prefilled Syringes Market for Blood Disorders: Analysis by Type of Chamber System
- 17.3.10.2.1. Single Chamber Prefilled Syringes Market for Blood Disorders
- 17.3.10.2.2. Dual Chamber Prefilled Syringes Market for Blood Disorders
- 17.3.10.3. Prefilled Syringes Market for Blood Disorders: Analysis by Type of Drug Molecule
- 17.3.10.3.1. Proteins Market for Blood Disorders
- 17.3.10.3.2. Small Molecules Market for Blood Disorders
- 17.3.10.4. Prefilled Syringes Market for Blood Disorders: Analysis by Geography
- 17.3.10.4.1. North America Market for Blood Disorders
- 17.3.10.4.2. Europe Market for Blood Disorders
- 17.3.10.4.3. Asia Pacific Market for Blood Disorders
- 17.3.10.4.4. Latin America Market for Blood Disorders
- 17.3.10.4.5. Middle East and Africa Market for Blood Disorders
- 17.3.10.1. Prefilled Syringes Market for Blood Disorders: Analysis by Type of Syringe Barrel Material
- 17.3.11. Prefilled Syringes Market for Oncological Disorders, 2022-2035
- 17.3.11.1. Prefilled Syringes Market for Oncological Disorders
- Analysis by Type of Syringe Barrel Material
- 17.3.11.1.1. Glass Barrel Prefilled Syringes Market for Oncological Disorders
- 17.3.11.1.2. Plastic Barrel Prefilled Syringes Market for Oncological Disorders
- 17.3.11.2. Prefilled Syringes Market for Oncological Disorders: Analysis by Type of Chamber System
- 17.3.11.2.1. Single Chamber Prefilled Syringes Market for Oncological Disorders
- 17.3.11.2.2. Dual Chamber Prefilled Syringes Market for Oncological Disorders
- 17.3.11.3. Prefilled Syringes Market for Oncological Disorders: Analysis by Type of Drug Molecule
- 17.3.11.3.1. Antibodies Market for Oncological Disorders
- 17.3.11.3.2. Proteins Market for Oncological Disorders
- 17.3.11.3.3. Peptides Market for Oncological Disorders
- 17.3.11.3.4. Small Molecules Market for Oncological Disorders
- 17.3.11.3.5. Vaccines Market for Oncological Disorders
- 17.3.11.4. Prefilled Syringes Market for Oncological Disorders: Analysis by Geography
- 17.3.11.4.1. North America Market for Oncological Disorders
- 17.3.11.4.2. Europe Market for Oncological Disorders
- 17.3.11.4.3. Asia Pacific Market for Oncological Disorders
- 17.3.11.4.4. Latin America Market for Oncological Disorders
- 17.3.11.4.5. Middle East and Africa Market for Oncological Disorders
- 17.3.11.1. Prefilled Syringes Market for Oncological Disorders
- 17.3.12. Prefilled Syringes Market for Metabolic Disorders, 2022-2035
- 17.3.12.1. Prefilled Syringes Market for Metabolic Disorders: Analysis by Type of Syringe Barrel Material
- 17.3.12.1.1. Glass Barrel Prefilled Syringes Market for Metabolic Disorders
- 17.3.12.1.2. Plastic Barrel Prefilled Syringes Market for Metabolic Disorders
- 17.3.12.2. Prefilled Syringes Market for Metabolic Disorders: Analysis by Type of Chamber System
- 17.3.12.2.1. Single Chamber Prefilled Syringes Market for Metabolic Disorders
- 17.3.12.2.2. Dual Chamber Prefilled Syringes Market for Metabolic Disorders
- 17.3.12.3. Prefilled Syringes Market for Metabolic Disorders: Analysis by Type of Drug Molecule
- 17.3.12.3.1. Proteins Market for Metabolic Disorders
- 17.3.12.3.2. Peptides Market for Metabolic Disorders
- 17.3.12.4. Prefilled Syringes Market for Metabolic Disorders: Analysis by Geography
- 17.3.12.4.1. North America Market for Metabolic Disorders
- 17.3.12.4.2. Europe Market for Metabolic Disorders
- 17.3.12.4.3. Asia Pacific Market for Metabolic Disorders
- 17.3.12.4.4. Latin America Market for Metabolic Disorders
- 17.3.12.4.5. Middle East and Africa Market for Metabolic Disorders
- 17.3.12.1. Prefilled Syringes Market for Metabolic Disorders: Analysis by Type of Syringe Barrel Material
- 17.3.13. Prefilled Syringes Market for Respiratory Disorders, 2022-2035
- 17.3.13.1. Prefilled Syringes Market for Respiratory Disorders: Analysis by Type of Syringe Barrel Material
- 17.3.13.1.1. Glass Barrel Prefilled Syringes Market for Respiratory Disorders
- 17.3.13.1.2. Plastic Barrel Prefilled Syringes Market for Respiratory Disorders
- 17.3.13.2. Prefilled Syringes Market for Respiratory Disorders: Analysis by Type of Chamber System
- 17.3.13.2.1. Single Chamber Prefilled Syringes Market for Respiratory Disorders
- 17.3.13.2.2. Dual Chamber Prefilled Syringes Market for Respiratory Disorders
- 17.3.13.3. Prefilled Syringes Market for Respiratory Disorders: Analysis by Type of Drug Molecule
- 17.3.13.3.1. Proteins Market for Respiratory Disorders
- 17.3.13.3.2. Peptides Market for Respiratory Disorders
- 17.3.13.4. Prefilled Syringes Market for Respiratory Disorders: Analysis by Geography
- 17.3.13.4.1. North America Market for Respiratory Disorders
- 17.3.13.4.2. Europe Market for Respiratory Disorders
- 17.3.13.4.3. Asia Pacific Market for Respiratory Disorders
- 17.3.13.4.4. Latin America Market for Respiratory Disorders
- 17.3.13.4.5. Middle East and Africa Market for Respiratory Disorders
- 17.3.13.1. Prefilled Syringes Market for Respiratory Disorders: Analysis by Type of Syringe Barrel Material
- 17.3.14. Prefilled Syringes Market for Ophthalmologic Disorders, 2022-2035
- 17.3.14.1. Prefilled Syringes Market for Ophthalmologic Disorders: Analysis by Type of Syringe Barrel Material
- 17.3.14.1.1. Glass Barrel Prefilled Syringes Market for Ophthalmologic Disorders
- 17.3.14.1.2. Plastic Barrel Prefilled Syringes Market for Ophthalmologic Disorders
- 17.3.14.2. Prefilled Syringes Market for Ophthalmologic Disorders: Analysis by Type of Chamber System
- 17.3.14.2.1. Single Chamber Prefilled Syringes Market for Ophthalmologic Disorders
- 17.3.14.2.2. Dual Chamber Prefilled Syringes Market for Ophthalmologic Disorders
- 17.3.14.3. Prefilled Syringes Market for Ophthalmologic Disorders: Analysis by Type of Drug Molecule
- 17.3.14.3.1. Proteins Market for Ophthalmologic Disorders
- 17.3.14.3.2. Peptides Market for Ophthalmologic Disorders
- 17.3.14.4. Prefilled Syringes Market for Ophthalmologic Disorders: Analysis by Geography
- 17.3.14.4.1. North America Market for Ophthalmologic Disorders
- 17.3.14.4.2. Europe Market for Ophthalmologic Disorders
- 17.3.14.4.3. Asia Pacific Market for Ophthalmologic Disorders
- 17.3.14.4.4. Latin America Market for Ophthalmologic Disorders
- 17.3.14.4.5. Middle East and Africa Market for Ophthalmologic Disorders
- 17.3.14.1. Prefilled Syringes Market for Ophthalmologic Disorders: Analysis by Type of Syringe Barrel Material
- 17.3.15. Prefilled Syringes Market for Other Disorders, 2022-2035
- 17.3.15.1. Prefilled Syringes Market for Other Disorders: Analysis by Type of Syringe Barrel Material
- 17.3.15.1.1. Glass Barrel Prefilled Syringes Market for Other Disorders
- 17.3.15.1.2. Plastic Barrel Prefilled Syringes Market for Other Disorders
- 17.3.15.2. Prefilled Syringes Market for Other Disorders: Analysis by Type of Chamber System
- 17.3.15.2.1. Single Chamber Prefilled Syringes Market for Other Disorders
- 17.3.15.2.2. Dual Chamber Prefilled Syringes Market for Other Disorders
- 17.3.15.3. Prefilled Syringes Market for Other Disorders: Analysis by Type of Drug Molecule
- 17.3.15.3.1. Antibodies Market for Other Disorders
- 17.3.15.3.2. Proteins Market for Other Disorders
- 17.3.15.3.3. Peptides Market for Other Disorders
- 17.3.15.3.4. Small Molecules Market for Other Disorders
- 17.3.15.4. Prefilled Syringes Market for Other Disorders: Analysis by Geography
- 17.3.15.4.1. North America Market for Other Disorders
- 17.3.15.4.2. Europe Market for Other Disorders
- 17.3.15.4.3. Asia Pacific Market for Other Disorders
- 17.3.15.4.4. Latin America Market for Other Disorders
- 17.3.15.4.5. Middle East and Africa Market for Other Disorders
- 17.3.15.1. Prefilled Syringes Market for Other Disorders: Analysis by Type of Syringe Barrel Material
- 17.3.1. Prefilled Syringes Market: Analysis by Therapeutic Area
18. KEY GROWTH DRIVERS
- 18.1. Chapter Overview
- 18.2. Rising Incidence of Chronic Diseases
- 18.3. Growing Preference for Self-Injection
- 18.4. Evolving Patient Demographics
- 18.5. Growth of Biologics and Biosimilars Market
- 18.6. Changing Pharmaceutical Strategies
- 18.7. Increasing Focus on Prevention of Needlestick Injuries
- 18.8. Prefilled Syringes in Autoinjectors and Pen Injectors
19. SWOT ANALYSIS
- 19.1. Chapter Overview
- 19.2. Strengths
- 19.3. Weaknesses
- 19.4. Opportunities
- 19.5. Threats
- 19.6. Concluding Remarks
20. PREFILLED SYRINGE COMPONENT MANUFACTURERS
- 20.1. Chapter Overview
- 20.2. List of Component Manufacturers
- 20.3. West Pharmaceutical
- 20.3.1. Company Overview
- 20.3.2. Financial Performance
- 20.3.3. Product Portfolio
- 20.3.4. Recent Developments and Future Outlook
- 20.4. Datwyler Sealing Solutions (a Part of Datwyler Group)
- 20.4.1. Company Overview
- 20.4.2. Financial Performance
- 20.4.3. Product Portfolio
- 20.4.4. Recent Developments
- 20.4.5 Future Outlook
- 20.5. Aptar Pharma (a Part of AptarGroup)
- 20.5.1. Company Overview
- 20.5.2. Financial Performance
- 20.5.3. Product Portfolio
- 20.5.4. Recent Developments
- 20.5.5. Future Outlook
- 20.6. Becton Dickinson
- 20.6.1. Company Overview
- 20.6.2. Financial Information
- 20.6.3. Product Portfolio
- 20.6.4. Recent Development and Future Outlook
- 20.7. Ompi (a Part of Stevanato Group)
- 20.7.1. Company Overview
- 20.7.2. Product Portfolio
- 20.7.3. Recent Developments and Future Outlook
- 20.8. Lonstroff (a Part of Sumitomo Rubber Industries)
- 20.8.1. Company Overview
- 20.8.2. Financial Performance
- 20.8.3. Product Portfolio
- 20.8.4. Recent Developments
- 20.8.5. Future Outlook
21. FILL / FINISH SERVICE PROVIDERS FOR PREFILLED SYRINGES
- 21.1. Chapter Overview
- 21.2. Fill / Finish Processing of Prefilled Syringes
- 21.2.1. Steps Involved in Fill / Finish Process
- 21.2.2. Methods of Filling and Stoppering of Prefilled Syringes
- 21.2.3. Prefilled Syringe Filling Technologies
- 21.3. Outsourcing Fill / Finish Operations
- 21.4. Growth Considerations
- 21.5. Prefilled Syringes: List of Fill / Finish Service Providers
- 21.5.1. Analysis by Year of Establishment
- 21.5.2. Analysis by Type of Drug Molecule and Location of Headquarters
- 21.5.3. Analysis by Scale of Operation
22. CASE STUDY: AUTOINJECTORS
- 22.1. Chapter Overview
- 22.2. Market Overview of Autoinjectors
- 22.3. Key Players
- 22.3.1. Elcam Medical (E3D Elcam Drug Delivery Devices)
- 22.3.1.1. Company Overview
- 22.3.1.2. Product Portfolio
- 22.3.2. Nuance Designs
- 22.3.2.1. Company Overview
- 22.3.2.2. Product Portfolio
- 22.3.3. Owen Mumford
- 22.3.3.1. Company Overview
- 22.3.3.2. Product Portfolio
- 22.3.4. Scandinavian Health Limited (SHL) Group
- 22.3.4.1. Company Overview
- 22.3.4.2. Product Portfolio
- 22.3.5. Union Medico
- 22.3.5.1. Company Overview
- 22.3.5.2. Product Portfolio
- 22.3.6. Ypsomed
- 22.3.6.1. Company Overview
- 22.3.6.2. Product Portfolio
- 22.3.1. Elcam Medical (E3D Elcam Drug Delivery Devices)
23. CONCLUDING REMARKS
- 23.1. Chapter Overview
- 23.2. Key Takeaways
24. INTERVIEW TRANSCRIPTS
- 24.1. Chapter Overview
- 24.2. Oval Medical Technologies
- 24.2.1. Company Snapshot
- 24.2.2. Interview Transcript: Matthew Young, Founder and Chief Technology Officer
- 24.3. Intas Pharmaceuticals
- 24.3.1. Company Snapshot
- 24.3.2. Interview Transcript: Kirti Maheshwari, Chief Operating Officer
- 24.4. IDT Biologika
- 24.4.1. Company Snapshot
- 24.4.2. Interview Transcript: Gregor Kawaletz, Ex-Chief Commercial Officer
- 24.5. West Pharmaceutical
- 24.5.1. Company Snapshot
- 24.5.2. Interview Transcript: Tibor Hlobik, Senior Director, Product Technology Services and Kevin Cancelliere, Ex-Director, Pharmaceutical Delivery Systems Marketing
- 24.6. Lonstroff
- 24.6.1. Company Snapshot
- 24.6.2. Interview Transcript: Marco Pederiva, Head of R&D and Product Strategy
- 24.7. IDEO
- 24.7.1. Company Snapshot
- 24.7.2. Interview Transcript: Jesse Fourt, Design Directors
- 24.8. Small-sized Medical Device Company
- 24.8.1. Interview Transcript: Anonymous, Chief Executive Officer
25. APPENDIX 1: TABULATED DATA
26. APPENDIX 2: LIST OF COMPANIES
List OfFigures
- Figure 3.1 Glass Barrel Prefilled Syringes: Advantages and Disadvantages
- Figure 3.2 Plastic Barrel Prefilled Syringes: Advantages and Disadvantages
- Figure 3.3 Critical Design Parameters of Prefilled Syringes
- Figure 3.4 Prefilled Syringe Manufacturing: Processes Involved
- Figure 4.1 Prefilled Syringes: Distribution by Type of Barrel Fabrication Material
- Figure 4.2 Prefilled Syringes: Distribution by Number of Barrel Chambers
- Figure 4.3 Prefilled Syringes: Distribution by Type of Needle System
- Figure 4.4 Prefilled Syringes: Distribution by Device Capacity
- Figure 4.5 Prefilled Syringe Developers: Distribution by Year of Establishment
- Figure 4.6 Prefilled Syringe Developers: Distribution by Company Size
- Figure 4.7 Prefilled Syringe Developers: Distribution by Location of Headquarters
- Figure 4.8 Prefilled Syringe Developers: Distribution by Company Size and Location of Headquarters
- Figure 4.9 Prefilled Syringe Developers: Distribution by Location of Manufacturing Facilities
- Figure 4.10 Technological Evolution of Prefilled Syringes
- Figure 5.1 Product Competitiveness Analysis: Glass Barrel Prefilled Syringes
- Figure 5.2 Product Competitiveness Analysis: Plastic Barrel Prefilled Syringes
- Figure 6.1 BD Medical: Annual Revenues, 2013 - 2021 (USD Billion)
- Figure 6.2 BD Medical: Distribution of Revenues by Business Segments (2021)
- Figure 6.3 Pfizer Hospital: Annual Revenues, 2011 - 2021 (USD Million)
- Figure 6.4 West Pharmaceutical: Annual Revenue, 2013 - 9M 2021 (USD Million)
- Figure 6.5 West Pharmaceutical: Distribution of Revenues by Business Segments, 2020
- Figure 6.6 West Pharmaceutical: Distribution of Revenues by Region, 2020
- Figure 6.7 Gerresheimer: Annual Revenues, 2013 - 2021 (EUR Million)
- Figure 6.8 Gerresheimer: Distribution of Revenues by Business Divisions (2020)
- Figure 6.9 Gerresheimer: Distribution of Revenue by Region (2020)
- Figure 6.10 Stevanato Group: Annual Revenues, 2018-2021 (EUR Million)
- Figure 6.11 Stevanato Group: Distribution of Revenues by Business Segments (2020)
- Figure 6.12 Stevanato Group: Distribution of Revenue by Region (2020)
- Figure 6.13 Schott: Annual Revenues, 2013-2021 (JPY Billion)
- Figure 6.14 Schott: Distribution of Revenues by Business Segments (2020)
- Figure 6.15 Schott: Distribution of Revenues by Region, (2020)
- Figure 6.16 Nipro: Annual Revenue, 2013-2021 (JPY Billion)
- Figure 6.17 Nipro: Distribution of Revenue by Business Segment (2020)
- Figure 6.18 Nipro: Distribution of Revenue by Region (2020)
- Figure 6.19 Terumo: Annual Revenues, 2012 - 9M 2021 (JPY Billion)
- Figure 6.20 Terumo: Distribution of Revenues by Business Divisions (2020)
- Figure 6.21 Terumo: Distribution of Revenue by Region (2020)
- Figure 6.22 Weigao: Annual Revenues, 2014 - 6M 2021 (RMB Billion)
- Figure 6.23 Weigao: Distribution of Revenue by Principle Products (2020)
- Figure 6.24 Weigao: Distribution of Revenue by Region (2020)
- Figure 7.1 Worldwide Evolution in Healthcare Safety Legislation
- Figure 8.1 Global Regulations Related to Prefilled Syringes
- Figure 8.2 Approval Pathway for Combination Products in the US
- Figure 8.3 Approval Pathway for Combination Products in Canada
- Figure 8.4 Pharmaceuticals and Medical Devices Agency: Review / Approval Process
- Figure 8.5 Approval Pathway for Combination Products in China
- Figure 9.1 Approved Prefilled Syringe Combination Products: Distribution by Type of Drug Molecule
- Figure 9.2 Approved Prefilled Syringe Combination Products: Distribution by Year
- Figure 9.3 Approved Prefilled Syringe Combination Products: Distribution by Geography
- Figure 9.4 Approved Prefilled Syringe Combination Products: Distribution by Route of Administration
- Figure 9.5 Approved Prefilled Syringe Combination Products: Distribution by Target Therapeutic Area
- Figure 9.6 Approved Prefilled Syringe Combination Products: Distribution by Other Dosage Forms
- Figure 9.7 Clinical Stage Prefilled Syringe Combination Products: Distribution by Type of Drug Molecule
- Figure 9.8 Clinical Stage Prefilled Syringe Combination Products: Distribution by Phase of Development
- Figure 9.9 Clinical Stage Prefilled Syringe Combination Products: Distribution by Route of Administration
- Figure 9.10 Approved Prefilled Syringe Combination Products: Distribution by Target Therapeutic Area
- Figure 9.11 Prefilled Syringe Combination Product Developers: Distribution by Year of Establishment
- Figure 9.12 Prefilled Syringe Combination Product Developers: Distribution by Company Size
- Figure 9.13 Prefilled Syringe Combination Product Developers: Distribution by Location of Headquarters
- Figure 9.14 Sales of Leading Drugs Available in Prefilled Syringes, 2021 (USD Billion)
- Figure 9.15 HUMIRA®: Approval Timeline (US, EU and Japan)
- Figure 9.16 HUMIRA®: Approval Timeline for Different Dosage Forms (US and EU)
- Figure 9.17 HUMIRA®: Annual Sales, 2003-9M 2021 (USD Million)
- Figure 9.18 Enbrel®: Approval Timeline (US and EU)
- Figure 9.19 Enbrel®: Approval Timeline for Different Dosage Forms (US and EU)
- Figure 9.20 Enbrel®: Annual Sales in the US and Canada, 2002-2021 (USD Million)
- Figure 9.21 Enbrel®: Annual Sales in other regions, 2010-2021 (USD Million)
- Figure 9.22 Enbrel®: Annual Sales in the US and Canada, 2002-2021 (USD Million)
- Figure 9.23 Enbrel®: Annual Sales in other regions, 2010-2021 (USD Million)
- Figure 11.1 Most Likely Partners for Prefilled Syringe Combination Product Developers in North America
- Figure 11.2 Likely Partners for Prefilled Syringe Combination Product Developers in North America
- Figure 11.3 Less Likely Partners for Prefilled Syringe Combination Product Developers in North America
- Figure 11.4 Least Likely Partners for Prefilled Syringe Combination Product Developers in North America
- Figure 11.5 Most Likely Partners for Prefilled Syringe Combination Product Developers in Europe
- Figure 11.6 Likely Partners for Prefilled Syringe Combination Product Developers in Europe
- Figure 11.7 Less Likely Partners for Prefilled Syringe Combination Product Developers in Europe
- Figure 11.8 Least Likely Partners for Prefilled Syringe Combination Product Developers in Europe
- Figure 11.9 Most Likely Partners for Prefilled Syringe Combination Product Developers in Asia-Pacific and RoW
- Figure 11.10 Likely Partners for Prefilled Syringe Combination Product Developers in Asia-Pacific and RoW
- Figure 11.11 Less Likely Partners for Prefilled Syringe Combination Product Developers in Asia-Pacific and RoW
- Figure 11.12 Least Likely Partners for Prefilled Syringe Combination Product Developers in Asia-Pacific and RoW
- Figure 12.1 Big Pharma Initiatives: Distribution by Therapeutic Area
- Figure 12.2 Big Pharma Initiatives for Autoimmune Disorders
- Figure 12.3 Big Pharma Initiatives for Autoimmune Disorders: Distribution by Target Indication
- Figure 12.4 Big Pharma Initiatives for Infectious Diseases
- Figure 12.5 Big Pharma Initiatives for Infectious Diseases: Distribution by Target Indication
- Figure 12.6 Big Pharma Initiatives for Oncological Disorders
- Figure 12.7 Big Pharma Initiatives for Oncological Disorders: Distribution by Target Indication
- Figure 12.8 Big Pharma Initiatives for Metabolic Disorders
- Figure 12.9 Big Pharma Initiatives for Metabolic Disorders: Distribution by Target Indication
- Figure 12.10 Big Pharma Initiatives for Neurological Disorders
- Figure 12.11 Big Pharma Initiatives for Neurological Disorders: Distribution by Target Indication
- Figure 12.12 Big Pharma Initiatives for Inflammatory Disorders
- Figure 12.13 Big Pharma Initiatives for Inflammatory Disorders: Distribution by Target Indication
- Figure 12.14 Big Pharma Initiatives for Orthopedic Disorders
- Figure 12.15 Big Pharma Initiatives for Orthopedic Disorders: Distribution by Target Indication
- Figure 12.16 Big Pharma Initiatives for Cardiovascular Disorders
- Figure 12.17 Big Pharma Initiatives for Cardiovascular Disorders: Distribution by Target Indication
- Figure 12.18 Big Pharma Initiatives for Respiratory Disorders
- Figure 12.19 Big Pharma Initiatives for Respiratory Disorders: Distribution by Target Indication
- Figure 12.20 Big Pharma Initiatives for Ophthalmic Disorders
- Figure 12.21 Big Pharma Initiatives for Ophthalmic Disorders: Distribution by Target Indication
- Figure 12.22 Big Pharma Initiatives for Other Disorders
- Figure 12.23 Big Pharma Initiatives: Distribution by Type of Drug Molecule
- Figure 12.24 Big Pharma Initiatives: Distribution by Antibodies
- Figure 12.25 Big Pharma Initiatives: Distribution by Vaccines
- Figure 12.26 Big Pharma Initiatives: Distribution by Proteins
- Figure 12.27 Big Pharma Initiatives: Distribution by Small Molecules
- Figure 12.28 Big Pharma Initiatives: Distribution by Peptides
- Figure 12.29 Big Pharma Initiatives: Distribution by Others
- Figure 13.1 Recent Developments: Distribution by Year of Initiative
- Figure 13.2 Recent Developments: Distribution by Type of Initiative
- Figure 13.3 Recent Developments: Distribution by Year and Type of Initiative
- Figure 13.4 Recent Developments: Distribution by Type of Expansion
- Figure 13.5 Recent Developments: Distribution by Region of Expansion
- Figure 13.6 Recent Developments: Distribution by Type of Partnership
- Figure 13.7 Recent Developments: Distribution by Location of Headquarters
- Figure 13.8 Most Active Players: Distribution by Number of Initiatives
- Figure 13.9 Recent Developments: Distribution of Players by Type of Initiative
- Figure 14.1 Patent Analysis: Distribution by Type of Patent
- Figure 14.2 Patent Analysis: Cumulative Distribution by Publication Year, 2017-2021
- Figure 14.3 Patent Analysis: Distribution by Type of Patent and Publication Year
- Figure 14.4 Patent Analysis: Distribution by Geography
- Figure 14.5 Patent Analysis: North America Scenario
- Figure 14.6 Patent Analysis: Asia-Pacific Scenario
- Figure 14.7 Patent Analysis: Distribution by CPC Symbols
- Figure 14.8 Patent Analysis: Distribution by Emerging Focus Area
- Figure 14.9 Most Active Players: Distribution by Number of Patents
- Figure 14.10 Key Inventors: Distribution by Number of Patents
- Figure 14.11 Patent Benchmarking Analysis: Terumo and Sumitomo Rubber
- Figure 14.12 Patent Benchmarking Analysis: Other Leading Players
- Figure 14.13 Patent Portfolio: Distribution by Patent Age
- Figure 14.14 Patent Valuation Analysis
- Figure 14.15 Overall Intellectual Property Portfolio: Distribution by Type of Organization
- Figure 17.1 Cost of Prefilled Syringes: Conservative, Base and Optimistic Scenarios, 2022-2035 (USD)
- Figure 17.2 Global Prefilled Syringes Market, 2022-2035 (Million Unit)
- Figure 17.3 Global Prefilled Syringes Market, 2022-2035 (USD Million)
- Figure 17.4 Prefilled Syringes Market: Distribution by Therapeutic Area, 2022-2035 (USD Million)
- Figure 17.5 Prefilled Syringes Market: Share of Autoimmune Disorders, 2022-2035 (USD Million)
- Figure 17.6 Prefilled Syringes Market: Share of Infectious Diseases, 2022-2035 (USD Million)
- Figure 17.7 Prefilled Syringes Market: Share of Neurological Disorders, 2022-2035 (USD Million)
- Figure 17.8 Prefilled Syringes Market: Share of Blood Disorders, 2022-2035 (USD Million)
- Figure 17.9 Prefilled Syringes Market: Share of Oncological Disorders, 2022-2035 (USD Million)
- Figure 17.10 Prefilled Syringes Market: Share of Respiratory Disorders, 2022-2035 (USD Million)
- Figure 17.11 Prefilled Syringes Market: Share of Cardiovascular Disorders, 2022-2035 (USD Million)
- Figure 17.12 Prefilled Syringes Market: Share of Metabolic Disorders, 2022-2035 (USD Million)
- Figure 17.13 Prefilled Syringes Market: Share of Ophthalmic Disorder, 2022-2035 (USD Million)
- Figure 17.14 Prefilled Syringes Market: Share of Orthopedic Disorders, 2022-2035 (USD Million)
- Figure 17.15 Prefilled Syringes Market: Share of Other Diseases, 2022-2035 (USD Million)
- Figure 17.16 Prefilled Syringes Market: Distribution by Type of Syringe Barrel Material, 2022 - 2035 (USD Million)
- Figure 17.17 Prefilled Syringes Market: Share of Glass Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.18 Prefilled Syringes Market: Share of Plastic Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.19 Prefilled Syringes Market: Distribution by Type of Chamber System, 2022 - 2035 (USD Million)
- Figure 17.20 Prefilled Syringes Market: Share of Single Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.21 Prefilled Syringes Market: Share of Dual Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.22 Prefilled Syringes Market: Distribution by Type of Drug Molecule, 2022-2035 (USD Million)
- Figure 17.23 Prefilled Syringes Market: Share of Antibodies, 2022-2035 (USD Million)
- Figure 17.24 Prefilled Syringes Market: Share of Proteins, 2022-2035 (USD Million)
- Figure 17.25 Prefilled Syringes Market: Share of Peptides, 2022-2035 (USD Million)
- Figure 17.26 Prefilled Syringes Market: Share of Small Molecules, 2022-2035 (USD Million)
- Figure 17.27 Prefilled Syringes Market: Share of Vaccines, 2022-2035 (USD Million)
- Figure 17.28 Prefilled Syringes Market: Distribution by Geography
- Figure 17.29 Prefilled Syringes Market: Share of North America, 2022-2035 (USD Million)
- Figure 17.30 Prefilled Syringes Market: Share of Europe, 2022-2035 (USD Million)
- Figure 17.31 Prefilled Syringes Market: Share of Asia Pacific, 2022-2035 (USD Million)
- Figure 17.32 Prefilled Syringes Market: Share of Latin America, 2022-2035 (USD Million)
- Figure 17.33 Prefilled Syringes Market: Share of Middle East and Africa, 2022-2035 (USD Million)
- Figure 17.34 Prefilled Syringes Market: Distribution by Specialty Syringes, 2022-2035 (USD Million)
- Figure 17.35 Prefilled Syringes Market for Autoimmune Disorders: Share of Glass Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.36 Prefilled Syringes Market for Autoimmune Disorders: Share of Plastic Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.37 Prefilled Syringes Market for Autoimmune Disorders: Share of Single Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.38 Prefilled Syringes Market for Autoimmune Disorders: Share of Dual Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.39 Prefilled Syringes Market for Autoimmune Disorders: Share of Antibodies, 2022-2035 (USD Million)
- Figure 17.40 Prefilled Syringes Market for Autoimmune Disorders: Share of Proteins, 2022-2035 (USD Million)
- Figure 17.41 Prefilled Syringes Market for Autoimmune Disorders: Share of North America, 2022-2035 (USD Million)
- Figure 17.42 Prefilled Syringes Market for Autoimmune Disorders: Share of Europe, 2022-2035 (USD Million)
- Figure 17.43 Prefilled Syringes Market for Autoimmune Disorders: Share of Asia Pacific, 2022-2035 (USD Million)
- Figure 17.44 Prefilled Syringes Market for Autoimmune Disorders: Share of Latin America, 2022-2035 (USD Million)
- Figure 17.45 Prefilled Syringes Market for Autoimmune Disorders: Share of Middle East and Africa, 2022-2035 (USD Million)
- Figure 17.46 Prefilled Syringes Market for Infectious Diseases: Share of Glass Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.47 Prefilled Syringes Market for Infectious Diseases: Share of Plastic Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.48 Prefilled Syringes Market for Infectious Diseases: Share of Single Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.49 Prefilled Syringes Market for Infectious Diseases: Share of Dual Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.50 Prefilled Syringes Market for Infectious Diseases: Share of Vaccines, 2022-2035 (USD Million)
- Figure 17.51 Prefilled Syringes Market for Infectious Diseases: Share of North America, 2022-2035 (USD Million)
- Figure 17.52 Prefilled Syringes Market for Infectious Diseases: Share of Europe, 2022-2035 (USD Million)
- Figure 17.53 Prefilled Syringes Market for Infectious Diseases: Share of Asia Pacific, 2022-2035 (USD Million)
- Figure 17.54 Prefilled Syringes Market for Infectious Diseases: Share of Latin America, 2022-2035 (USD Million)
- Figure 17.55 Prefilled Syringes Market for Infectious Diseases: Share of Middle East and Africa, 2022-2035 (USD Million)
- Figure 17.56 Prefilled Syringes Market for Neurological Disorders: Share of Glass Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.57 Prefilled Syringes Market for Neurological Disorders: Share of Plastic Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.58 Prefilled Syringes Market for Neurological Disorders: Share of Single Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.59 Prefilled Syringes Market for Neurological Disorders: Share of Dual Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.60 Prefilled Syringes Market for Neurological Disorders: Share of Antibodies, 2022-2035 (USD Million)
- Figure 17.61 Prefilled Syringes Market for Neurological Disorders: Share of North America, 2022-2035 (USD Million)
- Figure 17.62 Prefilled Syringes Market for Neurological Disorders: Share of Europe, 2022-2035 (USD Million)
- Figure 17.63 Prefilled Syringes Market for Neurological Disorders: Share of Asia Pacific, 2022-2035 (USD Million)
- Figure 17.64 Prefilled Syringes Market for Neurological Disorders: Share of Latin America, 2022-2035 (USD Million)
- Figure 17.65 Prefilled Syringes Market for Neurological Disorders: Share of Middle East and Africa, 2022-2035 (USD Million)
- Figure 17.66 Prefilled Syringes Market for Blood Disorders: Share of Glass Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.67 Prefilled Syringes Market for Blood Disorders: Share of Plastic Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.68 Prefilled Syringes Market for Blood Disorders: Share of Single Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.69 Prefilled Syringes Market for Blood Disorders: Share of Dual Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.70 Prefilled Syringes Market for Blood Disorders: Share of Proteins, 2022-2035 (USD Million)
- Figure 17.71 Prefilled Syringes Market for Blood Disorders: Share of Small Molecules, 2022-2035 (USD Million)
- Figure 17.72 Prefilled Syringes Market for Blood Disorders: Share of North America, 2022-2035 (USD Million)
- Figure 17.73 Prefilled Syringes Market for Blood Disorders: Share of Europe, 2022-2035 (USD Million)
- Figure 17.74 Prefilled Syringes Market for Blood Disorders: Share of Asia Pacific, 2022-2035 (USD Million)
- Figure 17.75 Prefilled Syringes Market for Blood Disorders: Share of Latin America, 2022-2035 (USD Million)
- Figure 17.76 Prefilled Syringes Market for Blood Disorders: Share of Middle East and Africa, 2022-2035 (USD Million)
- Figure 17.77 Prefilled Syringes Market for Oncological Disorders: Share of Glass Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.78 Prefilled Syringes Market for Oncological Disorders: Share of Plastic Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.79 Prefilled Syringes Market for Oncological Disorders: Share of Single Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.80 Prefilled Syringes Market for Oncological Disorders: Share of Dual Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.81 Prefilled Syringes Market for Oncological Disorders: Share of Antibodies, 2022-2035 (USD Million)
- Figure 17.82 Prefilled Syringes Market for Oncological Disorders: Share of Proteins, 2022-2035 (USD Million)
- Figure 17.83 Prefilled Syringes Market for Oncological Disorders: Share of Peptides, 2022-2035 (USD Million)
- Figure 17.84 Prefilled Syringes Market for Oncological Disorders: Share of North America, 2022-2035 (USD Million)
- Figure 17.85 Prefilled Syringes Market for Oncological Disorders: Share of Europe, 2022-2035 (USD Million)
- Figure 17.86 Prefilled Syringes Market for Oncological Disorders: Share of Asia Pacific, 2022-2035 (USD Million)
- Figure 17.87 Prefilled Syringes Market for Oncological Disorders: Share of Latin America, 2022-2035 (USD Million)
- Figure 17.88 Prefilled Syringes Market for Oncological Disorders: Share of Middle East and Africa, 2022-2035 (USD Million)
- Figure 17.89 Prefilled Syringes Market for Metabolic Disorders: Share of Glass Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.90 Prefilled Syringes Market for Metabolic Disorders: Share of Plastic Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.91 Prefilled Syringes Market for Metabolic Disorders: Share of Single Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.92 Prefilled Syringes Market for Metabolic Disorders: Share of Dual Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.93 Prefilled Syringes Market for Metabolic Disorders: Share of Peptides, 2022-2035 (USD Million)
- Figure 17.94 Prefilled Syringes Market for Metabolic Disorders: Share of North America, 2022-2035 (USD Million)
- Figure 17.95 Prefilled Syringes Market for Metabolic Disorders: Share of Europe, 2022-2035 (USD Million)
- Figure 17.96 Prefilled Syringes Market for Metabolic Disorders: Share of Asia Pacific, 2022-2035 (USD Million)
- Figure 17.97 Prefilled Syringes Market for Metabolic Disorders: Share of Latin America, 2022-2035 (USD Million)
- Figure 17.98 Prefilled Syringes Market for Metabolic Disorders: Share of Middle East and Africa, 2022-2035 (USD Million)
- Figure 17.99 Prefilled Syringes Market for Respiratory Disorders: Share of Glass Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.100 Prefilled Syringes Market for Respiratory Disorders: Share of Plastic Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.101 Prefilled Syringes Market for Respiratory Disorders: Share of Single Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.102 Prefilled Syringes Market for Respiratory Disorders: Share of Dual Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.103 Prefilled Syringes Market for Respiratory Disorders: Share of Peptides, 2022-2035 (USD Million)
- Figure 17.104 Prefilled Syringes Market for Respiratory Disorders: Share of North America, 2022-2035 (USD Million)
- Figure 17.105 Prefilled Syringes Market for Respiratory Disorders: Share of Europe, 2022-2035 (USD Million)
- Figure 17.106 Prefilled Syringes Market for Respiratory Disorders: Share of Asia Pacific, 2022-2035 (USD Million)
- Figure 17.107 Prefilled Syringes Market for Respiratory Disorders: Share of Latin America, 2022-2035 (USD Million)
- Figure 17.108 Prefilled Syringes Market for Respiratory Disorders: Share of Middle East and Africa, 2022-2035 (USD Million)
- Figure 17.109 Prefilled Syringes Market for Metabolic Disorders: Share of Glass Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.110 Prefilled Syringes Market for Metabolic Disorders: Share of Plastic Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.111 Prefilled Syringes Market for Metabolic Disorders: Share of Single Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.112 Prefilled Syringes Market for Metabolic Disorders: Share of Dual Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.113 Prefilled Syringes Market for Metabolic Disorders: Share of Antibodies, 2022-2035 (USD Million)
- Figure 17.114 Prefilled Syringes Market for Metabolic Disorders: Share of Peptide, 2022-2035 (USD Million)
- Figure 17.115 Prefilled Syringes Market for Metabolic Disorders: Share of Small Molecules, 2022-2035 (USD Million)
- Figure 17.116 Prefilled Syringes Market for Metabolic Disorders: Share of North America, 2022-2035 (USD Million)
- Figure 17.117 Prefilled Syringes Market for Metabolic Disorders: Share of Europe, 2022-2035 (USD Million)
- Figure 17.118 Prefilled Syringes Market for Metabolic Disorders: Share of Asia Pacific, 2022-2035 (USD Million)
- Figure 17.119 Prefilled Syringes Market for Metabolic Disorders: Share of Latin America, 2022-2035 (USD Million)
- Figure 17.120 Prefilled Syringes Market for Metabolic Disorders: Share of Middle East and Africa, 2022-2035 (USD Million)
- Figure 17.121 Prefilled Syringes Market for Cardiovascular Disorders: Share of Glass Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.122 Prefilled Syringes Market for Cardiovascular Disorders: Share of Plastic Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.123 Prefilled Syringes Market for Cardiovascular Disorders: Share of Single Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.124 Prefilled Syringes Market for Cardiovascular Disorders: Share of Dual Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.125 Prefilled Syringes Market for Cardiovascular Disorders: Share of Antibodies, 2022-2035 (USD Million)
- Figure 17.126 Prefilled Syringes Market for Cardiovascular Disorders: Share of Peptide, 2022-2035 (USD Million)
- Figure 17.127 Prefilled Syringes Market for Cardiovascular Disorders: Share of Small Molecules, 2022-2035 (USD Million)
- Figure 17.128 Prefilled Syringes Market for Cardiovascular Disorders: Share of North America, 2022-2035 (USD Million)
- Figure 17.129 Prefilled Syringes Market for Cardiovascular Disorders: Share of Europe, 2022-2035 (USD Million)
- Figure 17.130 Prefilled Syringes Market for Cardiovascular Disorders: Share of Asia Pacific, 2022-2035 (USD Million)
- Figure 17.131 Prefilled Syringes Market for Cardiovascular Disorders: Share of Latin America, 2022-2035 (USD Million)
- Figure 17.132 Prefilled Syringes Market for Cardiovascular Disorders: Share of Middle East and Africa, 2022-2035 (USD Million)
- Figure 17.133 Prefilled Syringes Market for Other Disorders: Share of Glass Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.134 Prefilled Syringes Market for Other Disorders: Share of Plastic Barrel Syringes, 2022-2035 (USD Million)
- Figure 17.135 Prefilled Syringes Market for Other Disorders: Share of Single Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.136 Prefilled Syringes Market for Other Disorders: Share of Dual Chamber Syringes, 2022-2035 (USD Million)
- Figure 17.137 Prefilled Syringes Market for Other Disorders: Share of Antibodies, 2022-2035 (USD Million)
- Figure 17.138 Prefilled Syringes Market for Other Disorders: Share of Peptide, 2022-2035 (USD Million)
- Figure 17.139 Prefilled Syringes Market for Other Disorders: Share of Small Molecules, 2022-2035 (USD Million)
- Figure 17.140 Prefilled Syringes Market for Other Disorders: Share of North America, 2022-2035 (USD Million)
- Figure 17.141 Prefilled Syringes Market for Other Disorders: Share of Europe, 2022-2035 (USD Million)
- Figure 17.142 Prefilled Syringes Market for Other Disorders: Share of Asia Pacific, 2022-2035 (USD Million)
- Figure 17.143 Prefilled Syringes Market for Other Disorders: Share of Latin America, 2022-2035 (USD Million)
- Figure 17.144 Prefilled Syringes Market for Other Disorders: Share of Middle East and Africa, 2022-2035 (USD Million)
- Figure 18.1 Diabetes: Country Wise Distribution of Prevalence and Mortality, 2017
- Figure 18.2 FDA Approved Biologics, 2009-2021
- Figure 18.3 Top Selling Biologics: Estimated Patent Expiry
- Figure 19.1 SWOT Analysis: Overview
- Figure 19.2 Prefilled Syringe Recalls: Distribution by Type of Error, 2003-2019
- Figure 19.3 Comparison of SWOT Factors: Harvey Ball Analysis
- Figure 20.1 Datwyler Group: Annual Revenues, 2013-2021 (CHF Billion)
- Figure 20.2 Aptar Pharma: Annual Revenues, 2013-H1 2019 (USD Billion)
- Figure 20.3 Sumitomo Rubber Industries: Annual Revenue, 2013-9M 2021(JPY Billion)
- Figure 20.4 Sumitomo Rubber Industries: Distribution of Revenues by Business Segments, (2020)
- Figure 21.1 Prefilled Syringe: Fill / Finish Process
- Figure 21.2 Prefilled Syringe Fill / Finish Service Providers: Distribution by Year of Establishment
- Figure 21.3 Prefilled Syringe Fill / Finish Service Providers: Distribution by Scale of Operation
- Figure 21.4 Prefilled Syringe Fill / Finish Service Providers: Distribution by Location of Headquarters and Type of Drug Molecule
- Figure 22.1 Elcam Medical: Product Portfolio
- Figure 22.2 Elcam Medical: Flexi-Q Autoinjectors
- Figure 22.3 Flexi-Q PFS: Key Components
- Figure 22.4 Flexi-Q PFS: Mechanism of Drug Delivery
- Figure 22.5 Flexi-Q DV: Key Components
- Figure 22.6 Flexi-Q DV: Mechanism of Drug Delivery
- Figure 22.7 Flexi-Q mMU: Key Components
- Figure 22.8 Flexi-Q eMU: Key Components
- Figure 22.9 Flexi-Q mMU / Flexi-Q eMU: Mechanism of Drug Delivery
- Figure 22.10 Flexi-Q EAI: Mechanism of Drug Delivery
- Figure 22.11 Owen Mumford: Product Portfolio
- Figure 22.12 Autoject Micro: Mechanism of Drug Delivery
- Figure 22.13 Autoject 2 Autoinjector: Mechanism of Drug Delivery
- Figure 22.14 Autoject Mini: Mechanism of Drug Delivery
- Figure 22.15 SHL Medical: Product Portfolio
- Figure 22.16 SHL Medical: Prefilled Syringe-based Autoinjectors
- Figure 22.17 SHL Medical: Cartridge-based Autoinjectors
- Figure 22.18 SHL Medical: Two-Step Autoinjectors
- Figure 22.19 SHL Medical Two-Step Autoinjectors: Mechanism of Drug Delivery
- Figure 22.20 Union Medico: 45? Autoinjector Portfolio
- Figure 22.21 Union Medico: Parts of 45?/ R Autoinjector
- Figure 22.22 Union Medico: Types of 45?/ R Autoinjector
- Figure 22.23 Union Medico: Types of 90? Autoinjectors
- Figure 22.24 Key Components of Autoinjectors
- Figure 22.25 Ypsomed: Annual Revenues, 2015- 2021 (CHF Million)
- Figure 22.26 Ypsomed: Sales by Business Divisions, 20XX (CHF Million, %)
- Figure 22.27 Ypsomed: Product Portfolio
- Figure 22.28 YpsoMate: Mechanism of Drug Delivery
- Figure 22.29 VarioJect: Mechanism of Drug Delivery
List OfTables
- Table 3.1 List of Drugs: Migration From Vials to Prefilled Syringe Format
- Table 3.2 Components of Prefilled Syringes
- Table 3.3 Classification of Prefilled Syringes
- Table 3.4 Advantages of Glass Barrel Fabrication Material
- Table 3.5 Glass Barrel Prefilled Syringe Recalls
- Table 3.6 Advantages of Plastic Polymer Barrel Fabrication Material
- Table 3.7 Steps Involved in Filling Process for Prefilled Syringe
- Table 4.1 Prefilled Syringes: List of Available / Under Development Devices
- Table 4.2 Prefilled Syringes: List of Developers
- Table 6.1 Key Manufacturers of Prefilled Syringes
- Table 6.2 Becton Dickinson: Future Outlook
- Table 6.3 West Pharmaceutical: Features of Daikyo Crystal Zenith Prefilled Syringe
- Table 6.4 West Pharmaceutical: Future Outlook
- Table 6.5 Gerresheimer: Features of Gx Glass Prefilled Syringe
- Table 6.6 Gerresheimer: Features of ClearJect Prefilled Syringe
- Table 6.7 Gerresheimer: Features of Gx RTF ClearJect Prefilled Syringe
- Table 6.8 Gerresheimer: Future Outlook
- Table 6.9 Ompi: Features of EZ-Fill Fina Prefilled Syringe
- Table 6.10 Ompi: Features of EZ-Fill Nexa Prefilled Syringe
- Table 6.11 Ompi: Features of EZ-Fill Alba Prefilled Syringe
- Table 6.12 Ompi: Future Outlook
- Table 6.13 Schott: Features of syriQ Prefilled Syringe
- Table 6.14 Schott: Features of TopPac Prefilled Syringe
- Table 6.15 Schott: Future Outlook
- Table 6.16 Nipro: Features of Prefilled Syringe
- Table 6.17 Taisei Kako: Features of VF-Syringes
- Table 6.18 Taisei Kako: Features of SIN-Syringes
- Table 6.19 Taisei Kako: Features of ClearJect Prefilled Syringes
- Table 6.20 Taisei Kako: Features of FULJECT Passive Type Syringes
- Table 6.21 Shandong Zibo: Features of Prefilled Syringes
- Table 6.22 Terumo: Features of Plajex Syringes
- Table 6.23 Terumo: Future Outlook
- Table 6.24 Vetter Pharma: Features of Lyo-Ject Glass Prefilled Syringe
- Table 6.25 Weigao: Features of WeGo Glass Prefilled Syringe
- Table 7.1 Add-On Safety Device Manufacturers
- Table 7.2 Integrated Prefilled Safety Device Manufacturers
- Table 8.1 US FDA Center for Drug and Device Approval
- Table 8.2 Regulatory Review Timelines in the US
- Table 8.3 Regulatory Bodies in EU5 Countries
- Table 8.4 Regulatory Device classification in Japan
- Table 8.5 Regulatory Review Timelines in Saudi Arabia
- Table 9.1 Prefilled Syringe Combination Products: List of Approved Drugs, 2013-2021
- Table 9.2 Prefilled Syringe Combination Products: Information on Approval Year and Key Geographies, 2013-2021
- Table 9.3 Prefilled Syringe Combination Products: Information on Dosage Strength
- Table 9.4 Prefilled Syringe Combination Products: List of Other Drug Delivery Solutions, 2013-2021
- Table 9.5 Prefilled Syringe Combination Products: List of Clinical Stage Drugs, 2013-2021
- Table 9.6 Prefilled Syringe Combination Products: List of Developers
- Table 9.7 Other Drugs Sold in Prefilled Syringes
- Table 10.1 List of Leading Drugs Approved for Autoimmune Disorders
- Table 10.2 List of Leading Drugs Available in Prefilled Syringes for Autoimmune Disorders
- Table 10.3 Information on Patent Expiry and Biosimilar Development Initiatives for Drugs Approved for Delivery via Prefilled Syringes for Autoimmune Disorders
- Table 10.4 List of Leading Drugs Approved for Antiviral Disorders
- Table 10.5 List of Leading Drugs Available in Prefilled Syringes Antiviral Drugs
- Table 10.6 Information on Patent Expiry and Biosimilar Development Initiatives for Drugs Approved for Delivery via Prefilled Syringes for Infectious Diseases
- Table 10.7 List of Leading Drugs Approved for Infectious Disorders
- Table 10.8 List of Leading Drugs Available in Prefilled Syringes for Infectious Disorders
- Table 10.9 List of Leading Drugs Approved for Neurological Disorders
- Table 10.10 List of Leading Drugs Available in Prefilled Syringes for Neurological Disorders
- Table 10.11 Information on Patent Expiry and Biosimilar Development Initiatives for Drugs Approved for Delivery via Prefilled Syringes for Neurological Disorders
- Table 10.12 List of Leading Drugs Approved for Metabolic Disorders
- Table 10.13 List of Leading Drugs Available in Prefilled Insulin Prefilled Syringes / Pen
- Table 10.14 Information on Patent Expiry and Biosimilar Development Initiatives for Drugs Approved for Delivery via Prefilled Syringes for Metabolic Disorders
- Table 11.1 Most Likely Marketed Drugs for Delivery via Prefilled Syringes
- Table 11.2 Likely Candidates Marketed Drugs for Delivery via Prefilled Syringes
- Table 11.3 Less Likely Candidates Marketed Drugs for Delivery via Prefilled Syringes
- Table 11.4 Least Likely Candidates Marketed Drugs for Delivery via Prefilled Syringes
- Table 11.5 Most Likely Clinical Stage Drug Candidates for Delivery via Prefilled Syringes
- Table 11.6 Likely Clinical Stage Drug Candidates for Delivery via Prefilled Syringes
- Table 11.7 Less Likely Clinical Stage Drug Candidates for Delivery via Prefilled Syringes
- Table 11.8 Least Likely Clinical Stage Drug Candidates for Delivery via Prefilled Syringes
- Table 13.1 Recent Developments: List of Initiatives, 2019 - 2021
- Table 14.1 Patent Analysis: List of Top CPC Symbols
- Table 14.2 Patent Analysis: Summary of Benchmarking Analysis
- Table 14.3 Patent Analysis: Various Categorizations based on Weighted Valuation Scores
- Table 14.4 Patent Analysis: List of Leading Patents (In Terms of Highest Relative Valuation)
- Table 15.1 Prefilled Flush Syringes Available in the Market
- Table 15.2 Drugs Available with Prefilled Diluent Syringe Systems
- Table 15.3 Contrast Agents Available in Prefilled Syringes
- Table 16.1 List of Dual / Multi-Chamber Prefilled Syringes
- Table 16.2 List of Drugs Marketed in Dual / Multi-Chamber Prefilled Syringes
- Table 16.3 Therapeutic Areas: Relative Share of Approved and Clinical Products with Prefilled Syringes
- Table 18.1 Fraction of Global Population above 60 years (in Millions), 2022, 2030 and 2050
- Table 18.2 Autoinjector Approvals, 2016-2021
- Table 18.3 List of Marketed Autoinjectors / Pen Injectors Available in Prefilled Syringe Format
- Table 19.1 List of Marketed/Pipeline Needleless Injection Devices
- Table 19.2 List of Prefilled Syringe Recalls, 2003-2021
- Table 20.1 Prefilled Syringe Component Manufacturers: List of Elastomeric Component Providers
- Table 20.2 Prefilled Syringes Elastomeric Container Closure Providers: List of Companies Profiled
- Table 20.3 West Pharmaceuticals: Company Overview
- Table 20.4 West Pharmaceuticals: Product Portfolio
- Table 20.5 Datwyler Sealing Solutions (Datwyler Group): Company Overview
- Table 20.6 Datwyler Sealing Solutions (Datwyler Group): Product Portfolio
- Table 20.7 Datwyler Sealing Solutions (Datwyler Group): Recent Developments and Future Outlook
- Table 20.8 Becton Dickinson: Company Overview
- Table 20.9 Becton Dickinson: Product Portfolio
- Table 20.10 Ompi: Company Overview
- Table 20.11 Ompi: Product Portfolio
- Table 20.12 Lonstroff: Company Overview
- Table 20.13 Sumitomo Rubber Industries: Company Overview
- Table 20.14 Lonstroff: Product Portfolio
- Table 21.1 List of Fill / Finish Service Providers
- Table 21.2 List of Autoinjector Devices (Using Prefilled Syringes)
- Table 21.3 YpsoMate Autoinjectors: Dimensions of Prefilled Syringes
- Table 21.4 Prefilled Syringes Market: Key Takeaways
- Table 23.1 Oval Medical Technologies: Key Highlights
- Table 23.2 Intas Pharmaceuticals: Key Highlights
- Table 23.3 IDT Biologika: Key Highlights
- Table 23.4 West Pharmaceutical: Key Highlights
- Table 23.5 Lonstroff: Key Highlights
- Table 24.1 Prefilled Syringes: Distribution by Type of Barrel Fabrication Material
- Table 24.2 Prefilled Syringes: Distribution by Number of Barrel Chambers
- Table 24.3 Prefilled Syringes: Distribution by Type of Needle System
- Table 24.4 Prefilled Syringes: Distribution by Packing Volume of Syringe
- Table 24.5 Prefilled Syringe Developers: Distribution by Year of Establishment
- Table 24.6 Prefilled Syringe Developers: Distribution by Company Size
- Table 24.7 Prefilled Syringe Developers: Distribution by Location of Headquarters
- Table 24.8 Prefilled Syringe Developers: Distribution by Company Size and Location of Headquarters
- Table 24.9 Prefilled Syringe Developers: Distribution by Location of Manufacturing Facilities
- Table 24.11 BD Medical: Annual Revenues, 2013 - 2021 (USD Billion)
- Table 24.12 BD Medical: Distribution of Revenue by Business Segments (2021)
- Table 24.13 Pfizer Hospital: Annual Revenues, 2011 - 2021 (USD Million)
- Table 24.14 West Pharmaceutical: Annual Revenue, 2013 - 9M 2021 (USD Million)
- Table 24.15 West Pharmaceutical: Distribution of Revenue by Business Segments, 2020
- Table 24.16 West Pharmaceutical: Distribution of Revenue by Region, 2020
- Table 24.17 Gerresheimer: Annual Revenues, 2013 - 2021 (EUR Million)
- Table 24.18 Gerresheimer: Distribution of Revenue by Business Divisions (2020)
- Table 24.19 Gerresheimer: Distribution of Revenue by Region (2020)
- Table 24.20 Stevanato Group: Annual Revenues, 2018-2021 (EUR Million)
- Table 24.21 Stevanato Group: Distribution of Revenue by Business Segments (2020)
- Table 24.22 Stevanato Group: Distribution of Revenue by Region (2020)
- Table 24.23 Schott: Annual Revenues, 2013-2021 (JPY Billion)
- Table 24.24 Schott: Distribution of Revenues by Business Segment (2020)
- Table 24.25 Schott: Distribution of Revenues by Region, (2020)
- Table 24.26 Nipro: Annual Revenue, 2013-2021 (JPY Billion)
- Table 24.27 Nipro: Distribution of Revenue by Business Segment (2020)
- Table 24.28 Nipro: Distribution of Revenue by Region (2020)
- Table 24.29 Terumo: Annual Revenues, 2012 - 9M 2021 (JPY Billion)
- Table 24.30 Terumo: Distribution of Revenue by Business Divisions (2020)
- Table 24.31 Terumo: Distribution of Revenue by Region (2020)
- Table 24.32 Weigao: Annual Revenues, 2014 - 6M 2021 (RMB Billion)
- Table 24.33 Weigao: Distribution of Revenue by Principle Products (2020)
- Table 24.34 Weigao: Distribution of Revenue by Region (2020)
- Table 24.35 Approved Prefilled Syringe Combination Products: Distribution by Type of Drug Molecule
- Table 24.36 Approved Prefilled Syringe Combination Products: Distribution by Year
- Table 24.37 Approved Prefilled Syringe Combination Products: Distribution by Geography
- Table 24.38 Approved Prefilled Syringe Combination Products: Distribution by Route of Administration
- Table 24.39 Approved Prefilled Syringe Combination Products: Distribution by Target Therapeutic Area
- Table 24.40 Approved Prefilled Syringe Combination Products: Distribution by Other Dosage Forms
- Table 24.41 Clinical Stage Prefilled Syringe Combination Products: Distribution by Type of Drug Molecule
- Table 24.42 Clinical Stage Prefilled Syringe Combination Products: Distribution by Phase of Development
- Table 24.43 Clinical Stage Prefilled Syringe Combination Products: Distribution by Route of Administration
- Table 24.44 Approved Prefilled Syringe Combination Products: Distribution by Target Therapeutic Area
- Table 24.45 Prefilled Syringe Combination Product Developers: Distribution by Year of Establishment
- Table 24.46 Prefilled Syringe Combination Product Developers: Distribution by Company Size
- Table 24.47 Prefilled Syringe Combination Product Developers: Distribution by Location of Headquarters
- Table 24.48 Sales of Leading Drugs Available in Prefilled Syringes, 2021 (USD Billion)
- Table 24.49 HUMIRA®: Annual Sales, 2003 - 9M 2021(USD Million)
- Table 24.50 Enbrel®: Annual Sales in the US and Canada, 2002-2021(USD Million)
- Table 24.51 Enbrel®: Annual Sales in Other regions, 2010-2021 (USD Million)
- Table 24.52 Enbrel®: Annual Sales in the US and Canada, 2002-2021 (USD Million)
- Table 24.53 Enbrel®: Annual Sales in other regions, 2010-2021 (USD Million)
- Table 24.54 Most Likely Partners for Prefilled Syringe Combination Product Developers in North America
- Table 24.55 Likely Partners for Prefilled Syringe Combination Product Developers in North America
- Table 24.56 Less Likely Partners for Prefilled Syringe Combination Product Developers in North America
- Table 24.57 Least Likely Partners for Prefilled Syringe Combination Product Developers in North America
- Table 24.58 Most Likely Partners for Prefilled Syringe Combination Product Developers in Europe
- Table 24.59 Likely Partners for Prefilled Syringe Combination Product Developers in Europe
- Table 24.60 Less Likely Partners for Prefilled Syringe Combination Product Developers in Europe
- Table 24.61 Least Likely Partners for Prefilled Syringe Combination Product Developers in Europe
- Table 24.62 Most Likely Partners for Prefilled Syringe Combination Product Developers in Asia-Pacific and RoW
- Table 24.63 Likely Partners for Prefilled Syringe Combination Product Developers in Asia-Pacific and RoW
- Table 24.64 Less Likely Partners for Prefilled Syringe Combination Product Developers in Asia-Pacific and RoW
- Table 24.65 Least Likely Partners for Prefilled Syringe Combination Product Developers in Asia-Pacific and RoW
- Table 24.66 Recent Developments: Distribution by Year of Initiative
- Table 24.67 Recent Developments: Distribution by Type of Initiative
- Table 24.68 Recent Developments: Distribution by Year and Type of Initiative
- Table 24.69 Recent Developments: Distribution by Type of Expansions
- Table 24.70 Recent Developments: Distribution by Region of Expansion
- Table 24.71 Recent Developments: Distribution by Focus of Expansion
- Table 24.72 Recent Developments: Distribution by Type of Partnership
- Table 24.73 Recent Developments: Distribution by Location of Headquarters
- Table 24.74 Most Active Players: Distribution by Number of Initiatives
- Table 24.75 Patent Analysis: Distribution by Type of Patent
- Table 24.76 Patent Analysis: Cumulative Distribution by Publication Year, 2017-2021
- Table 24.77 Patent Analysis: Distribution by Type of Patent and Publication Year
- Table 24.78 Patent Analysis: Distribution by Geography
- Table 24.79 Patent Analysis: North America Scenario
- Table 24.80 Patent Analysis: Europe Scenario
- Table 24.81 Patent Analysis: Asia-Pacific Scenario
- Table 24.82 Most Active Players: Distribution by Number of Patents
- Table 24.83 Key Inventors: Distribution by Number of Patents
- Table 24.84 Overall Intellectual Property Portfolio: Distribution by Type of Organization
- Table 24.85 Patent Portfolio: Distribution by Patent Age
- Table 24.86 Cost of Prefilled Syringes: Conservative, Base and Optimistic Scenarios, 2022-2035 (USD)
- Table 24.87 Global Prefilled Syringes Market, 2022-2035 (Million Unit)
- Table 24.88 Global Prefilled Syringes Market, 2022-2035 Conservative, Base and Optimistic Scenarios, (USD Million)
- Table 24.89 Prefilled Syringes Market: Distribution by Therapeutic Area, 2022-2035 (USD Million)
- Table 24.90 Prefilled Syringes Market: Share of Autoimmune Disorders, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.91 Prefilled Syringes Market: Share of Infectious Diseases, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.92 Prefilled Syringes Market: Share of Neurological Disorders, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.93 Prefilled Syringes Market: Share of Blood Disorders, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.94 Prefilled Syringes Market: Share of Oncological Disorders, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.95 Prefilled Syringes Market: Share of Respiratory Disorders, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.96 Prefilled Syringes Market: Share of Cardiovascular Disorders, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.97 Prefilled Syringes Market: Share of Metabolic Disorders, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.98 Prefilled Syringes Market: Share of Ophthalmic Disorder, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.99 Prefilled Syringes Market: Share of Orthopedic Disorders, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.100 Prefilled Syringes Market: Share of Other Diseases, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.101 Prefilled Syringes Market: Distribution by Type of Syringe Barrel Material, 2022 - 2035 (USD Million)
- Table 24.102 Prefilled Syringes Market: Share of Glass Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.103 Prefilled Syringes Market: Share of Plastic Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.104 Prefilled Syringes Market: Distribution by Type of Chamber System, 2022-2035 (USD Million) (USD Million)
- Table 24.105 Prefilled Syringes Market: Share of Single Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.106 Prefilled Syringes Market: Share of Dual Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.107 Prefilled Syringes Market: Distribution by Type of Drug Molecule, 2022-2035 (USD Million) (USD Million)
- Table 24.108 Prefilled Syringes Market: Share of Antibodies, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.109 Prefilled Syringes Market: Share of Proteins, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.110 Prefilled Syringes Market: Share of Peptides, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.111 Prefilled Syringes Market: Share of Small Molecules, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.112 Prefilled Syringes Market: Share of Vaccines, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.113 Prefilled Syringes Market: Distribution by Geographical Regions
- Table 24.114 Prefilled Syringes Market: Share of North America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.115 Prefilled Syringes Market: Share of Europe, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.116 Prefilled Syringes Market: Share of Asia Pacific, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.117 Prefilled Syringes Market: Share of Latin America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.118 Prefilled Syringes Market: Share of Middle East and Africa, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.119 Prefilled Syringes Market: Distribution by Specialty Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.120 Prefilled Syringes Market for Autoimmune Disorders: Share of Glass Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.121 Prefilled Syringes Market for Autoimmune Disorders: Share of Plastic Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.122 Prefilled Syringes Market for Autoimmune Disorders: Share of Single Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.123 Prefilled Syringes Market for Autoimmune Disorders: Share of Dual Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.124 Prefilled Syringes Market for Autoimmune Disorders: Share of Antibodies, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.125 Prefilled Syringes Market for Autoimmune Disorders: Share of Proteins, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.126 Prefilled Syringes Market for Autoimmune Disorders: Share of North America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.127 Prefilled Syringes Market for Autoimmune Disorders: Share of Europe, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.128 Prefilled Syringes Market for Autoimmune Disorders: Share of Asia Pacific, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.129 Prefilled Syringes Market for Autoimmune Disorders: Share of Latin America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.130 Prefilled Syringes Market for Autoimmune Disorders: Share of Middle East and Africa, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.131 Prefilled Syringes Market for Infectious Disorders: Share of Glass Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.132 Prefilled Syringes Market for Infectious Disorders: Share of Plastic Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.133 Prefilled Syringes Market for Infectious Disorders: Share of Single Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.134 Prefilled Syringes Market for Infectious Disorders: Share of Dual Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.135 Prefilled Syringes Market for Infectious Disorders: Share of Vaccines, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.136 Prefilled Syringes Market for Infectious Disorders: Share of North America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.137 Prefilled Syringes Market for Infectious Disorders: Share of Europe, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.138 Prefilled Syringes Market for Infectious Disorders: Share of Asia Pacific, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.139 Prefilled Syringes Market for Infectious Disorders: Share of Latin America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.140 Prefilled Syringes Market for Infectious Disorders: Share of Middle East and Africa, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.141 Prefilled Syringes Market for Neurological Disorders: Share of Glass Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.142 Prefilled Syringes Market for Neurological Disorders: Share of Plastic Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.143 Prefilled Syringes Market for Neurological Disorders: Share of Single Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.144 Prefilled Syringes Market for Neurological Disorders: Share of Dual Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.145 Prefilled Syringes Market for Neurological Disorders: Share of Antibodies, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.146 Prefilled Syringes Market for Neurological Disorders: Share of North America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.147 Prefilled Syringes Market for Neurological Disorders: Share of Europe, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.148 Prefilled Syringes Market for Neurological Disorders: Share of Asia Pacific, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.149 Prefilled Syringes Market for Neurological Disorders: Share of Latin America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.150 Prefilled Syringes Market for Neurological Disorders: Share of Middle East and Africa, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.151 Prefilled Syringes Market for Blood Disorders: Share of Glass Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.152 Prefilled Syringes Market for Blood Disorders: Share of Plastic Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.153 Prefilled Syringes Market for Blood Disorders: Share of Single Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.154 Prefilled Syringes Market for Blood Disorders: Share of Dual Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.155 Prefilled Syringes Market for Blood Disorders: Share of Proteins, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.156 Prefilled Syringes Market for Blood Disorders: Share of Small Molecules, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.157 Prefilled Syringes Market for Blood Disorders: Share of North America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.158 Prefilled Syringes Market for Blood Disorders: Share of Europe, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.159 Prefilled Syringes Market for Blood Disorders: Share of Asia Pacific, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.160 Prefilled Syringes Market for Blood Disorders: Share of Latin America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.161 Prefilled Syringes Market for Blood Disorders: Share of Middle East and Africa, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.162 Prefilled Syringes Market for Oncological Disorders: Share of Glass Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.163 Prefilled Syringes Market for Oncological Disorders: Share of Plastic Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.164 Prefilled Syringes Market for Oncological Disorders: Share of Single Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.165 Prefilled Syringes Market for Oncological Disorders: Share of Dual Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.166 Prefilled Syringes Market for Oncological Disorders: Share of Antibodies, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.167 Prefilled Syringes Market for Oncological Disorders: Share of Proteins, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.168 Prefilled Syringes Market for Oncological Disorders: Share of Peptide, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.169 Prefilled Syringes Market for Oncological Disorders: Share of North America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.170 Prefilled Syringes Market for Oncological Disorders: Share of Europe, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.171 Prefilled Syringes Market for Oncological Disorders: Share of Asia Pacific, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.172 Prefilled Syringes Market for Oncological Disorders: Share of Latin America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.173 Prefilled Syringes Market for Oncological Disorders: Share of Middle East and Africa, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.174 Prefilled Syringes Market for Metabolic Disorders: Share of Glass Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.175 Prefilled Syringes Market for Metabolic Disorders: Share of Plastic Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.176 Prefilled Syringes Market for Metabolic Disorders: Share of Single Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.177 Prefilled Syringes Market for Metabolic Disorders: Share of Dual Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.178 Prefilled Syringes Market for Metabolic Disorders: Share of Peptides, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.179 Prefilled Syringes Market for Metabolic Disorders: Share of North America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.180 Prefilled Syringes Market for Metabolic Disorders: Share of Europe, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.181 Prefilled Syringes Market for Metabolic Disorders: Share of Asia Pacific, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.182 Prefilled Syringes Market for Metabolic Disorders: Share of Latin America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.183 Prefilled Syringes Market for Metabolic Disorders: Share of Middle East and Africa, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.184 Prefilled Syringes Market for Respiratory Disorders: Share of Glass Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.185 Prefilled Syringes Market for Respiratory Disorders: Share of Plastic Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.186 Prefilled Syringes Market for Respiratory Disorders: Share of Single Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.187 Prefilled Syringes Market for Respiratory Disorders: Share of Dual Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.188 Prefilled Syringes Market for Respiratory Disorders: Share of Peptides, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.189 Prefilled Syringes Market for Respiratory Disorders: Share of North America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.190 Prefilled Syringes Market for Respiratory Disorders: Share of Europe, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.191 Prefilled Syringes Market for Respiratory Disorders: Share of Asia Pacific, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.192 Prefilled Syringes Market for Respiratory Disorders: Share of Latin America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.193 Prefilled Syringes Market for Respiratory Disorders: Share of Middle East and Africa, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.194 Prefilled Syringes Market for Metabolic Disorders: Share of Glass Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.195 Prefilled Syringes Market for Metabolic Disorders: Share of Plastic Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.196 Prefilled Syringes Market for Metabolic Disorders: Share of Single Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.197 Prefilled Syringes Market for Metabolic Disorders: Share of Dual Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.198 Prefilled Syringes Market for Metabolic Disorders: Share of Antibodies, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.199 Prefilled Syringes Market for Metabolic Disorders: Share of Peptide, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.200 Prefilled Syringes Market for Metabolic Disorders: Share of Small Molecules, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.201 Prefilled Syringes Market for Metabolic Disorders: Share of North America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.202 Prefilled Syringes Market for Metabolic Disorders: Share of Europe, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.203 Prefilled Syringes Market for Metabolic Disorders: Share of Asia Pacific, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.204 Prefilled Syringes Market for Metabolic Disorders: Share of Latin America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.205 Prefilled Syringes Market for Metabolic Disorders: Share of Middle East and Africa, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.206 Prefilled Syringes Market for Cardiovascular Disorders: Share of Glass Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.207 Prefilled Syringes Market for Cardiovascular Disorders: Share of Plastic Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.208 Prefilled Syringes Market for Cardiovascular Disorders: Share of Single Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.209 Prefilled Syringes Market for Cardiovascular Disorders: Share of Dual Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.210 Prefilled Syringes Market for Cardiovascular Disorders: Share of Antibodies, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.211 Prefilled Syringes Market for Cardiovascular Disorders: Share of Peptide, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.212 Prefilled Syringes Market for Cardiovascular Disorders: Share of Small Molecules, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.213 Prefilled Syringes Market for Cardiovascular Disorders: Share of North America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.214 Prefilled Syringes Market for Cardiovascular Disorders: Share of Europe, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.215 Prefilled Syringes Market for Cardiovascular Disorders: Share of Asia Pacific, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.216 Prefilled Syringes Market for Cardiovascular Disorders: Share of Latin America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.217 Prefilled Syringes Market for Cardiovascular Disorders: Share of Middle East and Africa, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.218 Prefilled Syringes Market for Other Disorders: Share of Glass Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.219 Prefilled Syringes Market for Other Disorders: Share of Plastic Barrel Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.220 Prefilled Syringes Market for Other Disorders: Share of Single Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.221 Prefilled Syringes Market for Other Disorders: Share of Dual Chamber Syringes, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.222 Prefilled Syringes Market for Other Disorders: Share of Antibodies, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.223 Prefilled Syringes Market for Other Disorders: Share of Peptide, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.224 Prefilled Syringes Market for Other Disorders: Share of Small Molecules, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.225 Prefilled Syringes Market for Other Disorders: Share of North America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.226 Prefilled Syringes Market for Other Disorders: Share of Europe, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.227 Prefilled Syringes Market for Other Disorders: Share of Asia Pacific, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.228 Prefilled Syringes Market for Other Disorders: Share of Latin America, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.229 Prefilled Syringes Market for Other Disorders: Share of Middle East and Africa, Conservative, Base and Optimistic Scenarios, 2022-2035 (USD Million)
- Table 24.230 Diabetes: Country Wise Distribution of Prevalence and Mortality, 2017
- Table 24.231 FDA Approved Biologics, 2009-20XX
- Table 24.232 Top Selling Biologics: Estimated Patent Expiry
- Table 24.234 Aptar Pharma: Annual Revenues, 2013 - H1 20XX (USD Billion)
- Table 24.233 Datwyler Group: Annual Revenues, 2013 - 2021 (CHF Billion)
- Table 24.235 Sumitomo Rubber Industries: Annual Revenue, 2013 - 9M 2021(JPY Billion)
- Table 24.236 Sumitomo Rubber Industries: Distribution of Revenue by Business Segment, (2020)
- Table 24.237 Prefilled Syringe Fill / Finish Service Providers: Distribution by Year of Establishment
- Table 24.238 Prefilled Syringe Fill / Finish Service Providers: Distribution by Scale of Operation
List OfCompanies
The following companies and organizations have been mentioned in the report
- 1. 89bio
- 2. Abbott Laboratories
- 3. AbbVie
- 4. Accord Healthcare
- 5. Adamis Pharmaceuticals
- 6. Adimmune
- 7. Advance Pharmalab
- 8. Aenova
- 9. AEON Biopharma
- 10. Ajinomoto Bio-Pharma Services
- 11. Akums Drugs & Pharmaceuticals
- 12. Alcami
- 13. Alder BioPharmaceuticals
- 14. Alexion Pharmaceuticals
- 15. Alfasigma
- 16. Alkermes
- 17. Allakos
- 18. Allergan
- 19. Alvogen
- 20. Alvotech
- 21. Amgen
- 22. Amphastar Pharmaceuticals
- 23. Amryt
- 24. Andes Biotechnologies
- 25. AnGes
- 26. Anhui Huafeng Pharmaceutical Rubber
- 27. ApcinteX (Acquired by Centessa Pharmaceuticals)
- 28. Apotek Produktion & Laboratorier
- 29. Apotex
- 30. Aptar Pharma
- 31. Araclon Biotech
- 32. Arecor Therapeutics
- 33. argenx
- 34. Arrowhead Pharmaceuticals
- 35. ARTE
- 36. Asarina Pharma
- 37. Ascendis Pharma
- 38. Ashvattha Therapeutics
- 39. Aspen Pharmacare
- 40. Aspire Life Sciences
- 41. ASST Fatebenefratelli Sacco
- 42. Astellas Pharma
- 43. Astex Pharmaceuticals
- 44. AstraZeneca
- 45. Atlant Clinical
- 46. Atridia
- 47. B. Braun
- 48. Baxter BioPharma Solutions
- 49. Bayer
- 50. Becton Dickinson
- 51. Berkshire Sterile Manufacturing
- 52. Beximco Pharma
- 53. Bharat Biotech
- 54. BIOCAD
- 55. Biocon
- 56. Biogen
- 57. Bioinova
- 58. Birgi Mefar
- 59. Boehringer Ingelheim
- 60. Bone Therapeutics
- 61. Bristol Myers Squibb
- 62. Caliway Biopharmaceuticals
- 63. Camurus
- 64. CARBOGEN AMCIS
- 65. Cardinal Health
- 66. CardioVascular BioTherapeutics
- 67. Catalent Biologics
- 68. Celgene
- 69. Celldex Therapeutics
- 70. Celltrion Healthcare
- 71. Cenexi
- 72. Centrexion Therapeutics
- 73. Chong Kun Dang Pharmaceutical
- 74. Chugai Pharmaceutical
- 75. CinnaGen
- 76. Ciron
- 77. Clinuvel Pharmaceuticals
- 78. Coherus BioSciences
- 79. Consort Medical
- 80. CordenPharma
- 81. Credence MedSystems
- 82. CSL Behring
- 83. Cumberland Pharmaceuticals
- 84. Curia (formerly known as AMRI)
- 85. Cutia Therapeutics
- 86. Cycle Pharmaceuticals
- 87. Daewoong Pharmaceutical
- 88. Daiichi Sankyo
- 89. Danyang Jincheng Medical Rubber & Plastics
- 90. Data Management 365
- 91. Datwyler
- 92. Delpharm
- 93. Dicerna Pharmaceuticals
- 94. Dynavax Technologies
- 95. Edge Pharma
- 96. Eiger BioPharmaceuticals
- 97. Eisai
- 98. Eli Lilly and Company
- 99. Emcure Pharmaceuticals
- 100. EMD Serono
- 101. Emergent BioSolutions
- 102. Endocard
- 103. Ethypharm
- 104. Eureka Forbes
- 105. Eurofarma
- 106. EVER Pharma
- 107. Express Service Import Export
- 108. Ferring Pharmaceuticals
- 109. Foresee Pharmaceuticals
- 110. Fresenius Kabi
- 111. Fujian Shengdi Pharmaceutical
- 112. Fujifilm Toyama Chemical
- 113. Galaxy Pharmaceuticals
- 114. GC Pharma
- 115. GemVax & KAEL
- 116. Genentech
- 117. Generon
- 118. Genmab
- 119. Gerresheimer
- 120. Gilead Sciences
- 121. Gland Pharma
- 122. GlaxoSmithKline
- 123. Glenmark Pharmaceuticals
- 124. Grifols Therapeutics
- 125. H K Zion Industry
- 126. Hanmi Pharmaceutical
- 127. Harbour BioMed
- 128. Healthcare Pharmaceuticals
- 129. Heptares Therapeutics
- 130. Heron Therapeutics
- 131. Hetero Drugs
- 132. HK Surgical
- 133. Hoffmann-La Roche
- 134. Institut Biochimique
- 135. IDT Biologika
- 136. IlYang Pharmaceuticals
- 137. ImmuneMed
- 138. ImmunityBio
- 139. Inhibrx
- 140. Injecto
- 141. Inmunotek
- 142. Innovent Biologics
- 143. Intas Pharmaceuticals
- 144. Intra-Cellular Therapies
- 145. Ipsen
- 146. IQVIA
- 147. Italfarmaco
- 148. Jain Rubbers
- 149. Janssen Pharmaceuticals
- 150. JCR Pharmaceuticals
- 151. Jiangsu HengRui Medicine
- 152. Jiangsu Hualan New Pharmaceutical Material
- 153. Jiangxi Qingfeng Pharmaceutical
- 154. Jiangyin Hongmeng Rubber Plastic Product
- 155. Johnson & Johnson
- 156. Kastle Therapeutics
- 157. Kemwell Biopharma
- 158. Keymed Biosciences
- 159. Kiniksa Pharmaceuticals
- 160. Kitasato Daiichi Sankyo Vaccine
- 161. Kymab (Acquired by Sanofi)
- 162. Kyowa Hakko Kirin
- 163. Labcorp
- 164. Laboratoire AGUETTANT
- 165. Salvat Laboratories
- 166. LEO Pharma
- 167. Lundbeck
- 168. Luye Pharma Group
- 169. Lyophilization Services of New England (LSNE)
- 170. Mapi Pharma
- 171. Maruishi Pharmaceutical
- 172. Medicago
- 173. Medicalchain
- 174. Medline
- 175. Medica Scientia Innovation Research (MEDSIR)
- 176. Megalabs
- 177. Merck
- 178. Millennium Pharmaceuticals
- 179. Mitsubishi Gas Chemical
- 180. Momenta Pharmaceuticals
- 181. MorphoSys
- 182. Mylan
- 183. Nanexa
- 184. Nektar Therapeutics
- 185. NeuroDerm
- 186. Nexus Pharmaceuticals
- 187. Ningbo Zhengli Pharmaceutical Packaging
- 188. Nippon Organon
- 189. Nipro PharmaPackaging
- 190. Novartis
- 191. Novo Nordisk
- 192. Octapharma
- 193. Oncorus
- 194. Otsuka Pharmaceutical
- 195. Outlook Therapeutics
- 196. PapiVax Biotech
- 197. PCI Pharma Services
- 198. Paige Biopharmaceutical
- 199. Peptron
- 200. Pfizer
- 201. PharmaEssentia
- 202. PHC Injection Device Technologies
- 203. Piramal Pharma
- 204. Pisa Farmaceutica
- 205. Plas-Tech Engineering
- 206. Pluristem Therapeutics
- 207. Polpharma Biologics
- 208. Primequal
- 209. Propella Therapeutics
- 210. PTC Therapeutics
- 211. Qindao Huaren Medical
- 212. RAUMEDIC
- 213. Regeneron
- 214. Rejuven Dermaceutical
- 215. Reliance Life Sciences
- 216. Rentschler Biopharma
- 217. RHEACELL
- 218. Roche
- 219. Laboratorios Farmaceuticos Rovi
- 220. R-Pharm
- 221. Sagar Rubber Products
- 222. Samsung Bioepis
- 223. Samsung BioLogics
- 224. Samsung Medical Rubber
- 225. Sandoz
- 226. Sanofi
- 227. Scancell
- 228. SCHOTT
- 229. SciPharm
- 230. Secura Bio
- 231. Seqirus
- 232. Serina Therapeutics
- 233. Serum Institute of India
- 234. Sewa Medicals
- 235. Shan Dong Boan Biotechnology (Acquired by Luye Pharma)
- 236. Shandong Jihai Medical Technology
- 237. Shandong Pharmaceutical Glass
- 238. Shandong Weigao
- 239. Shandong Zibo Minkang Pharmaceutical Packaging
- 240. Shanghai Hengrui Pharmaceutical
- 241. Sheng Zou Rubber & Plastics
- 242. Shin Yan Sheno Precision Industrial
- 243. Shire
- 244. Shrinath Products (A Divison of Elmer Products)
- 245. Siegfried Holding
- 246. Splash Pharmaceuticals
- 247. Square Pharmaceuticals
- 248. SRI International
- 249. SRK Pharmaceuticals
- 250. STADA Arzneimittel
- 251. Stealth BioTherapeutics
- 252. Sumitomo Rubber Industries
- 253. Sun Pharmaceutical Industries
- 254. Sunshine Guojian
- 255. Surecare Pharma
- 256. Swedish Orphan Biovitrum
- 257. Taizhou Kanglong Pharmaceutical Packing
- 258. TaiMed Biologics
- 259. Taisei Kako
- 260. Taj Life Sciences
- 261. Taj Pharmaceuticals
- 262. Takeda
- 263. Talecris Biotherapeutics
- 264. Terumo
- 265. Tetherex Pharmaceuticals
- 266. Teva Pharmaceuticals
- 267. The Plasticoid Company
- 268. Tip-Top
- 269. Tolmar
- 270. Toray Industries
- 271. Transcoject
- 272. Trinomab Biotech
- 273. UCB Biopharma
- 274. Closed Joint Stock Company (CJSC) Unimed Laboratories
- 275. United BioPharma
- 276. Universal Medicap
- 277. US WorldMeds
- 278. Vetter Pharma
- 279. VHB Medi Sciences
- 280. Vighnesh Rubbers
- 281. ViiV Healthcare
- 282. VITARIS
- 283. Weidmann Medical Technology
- 284. West Pharmaceutical
- 285. WuXi Biologics
- 286. Xbiotech
- 287. Xencor
- 288. Xeris Biopharma
- 289. YL Biologics
- 290. Yuhan
- 291. Zealand Pharma
- 292. Zen Pharma